Manuscript accepted on : December 12, 2010
Published online on: 28-12-2010
R. Pratap Chandran* and V. P. Potty1
1Central Tuber Crops Research Institute, Thiruvananthapuram - 695 017 India.
2Cashew Export Promotion Council Lab and Technology Division, Mundakkal East, Kollam - 691 001 India.
Corresponding Author E-mail:drpratapchandran@yahoo.co.in
ABSTRACT: Arbuscular mycorrhizal (AM) fungi are obligate biotrophs and they cannot complete their life cycle in the absence of a host plant root. AM fungus, Glomus microcarpum was successfully co-cultivated in transgenic hairy roots of I. batatas (variety S1010) initiated through the mediation of Agrobacterium rhizogenes ATCC 15834. Murashige and Skoog (MS) medium composition was modified to support maximum hairy root growth and MS vitamins were replaced with B5 vitamins for better growth of hairy roots. Temperature, pH and light regimens were standardized for optimum hairy root growth and AM colonization. Eighty percent mycorrhizal colonization was observed after 20 days of co-cultivation in modified MS medium in petri dish. Various stages of mycorrhizal infection, pre-infection, penetration, vesicle/arbuscule formation were observed and viability of fungal structures was also studied. This technique can be used for the large scale production of monospecific culture of AM fungi.
KEYWORDS: Agrobacterium rhizogenes; Arbuscular mycorrhizal fungi; colonization; Glomus microcarpum
Download this article as:| Copy the following to cite this article: Chandran R. P, Potty V. P. Colonization of Glomus Microcarpum in Ri T-DNA Transformed Roots of Ipomoea Batatas (S1010) in Synthetic Medium. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Chandran R. P, Potty V. P. Colonization of Glomus Microcarpum in Ri T-DNA Transformed Roots of Ipomoea Batatas (S1010) in Synthetic Medium. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9373 |
Introduction
The AM fungal association is geographically ubiquitous, occurring in plants from arctic to tropical regions, over a broad ecological range from aquatic to desert environments. The AM fungus plant association is a mutualistic beneficial event, in which plant supplies the fungus with carbon from its fixed photosynthates, while the fungus assists plants in its uptake of phosphate and other mineral nutrients from the soil1. The AM fungi may produce an extensive network of extrametrical hyphae2 and can significantly increase phosphorous inflow rates of plants they colonize3. The harmonious symbiotic relationship is reflected in the obligate biotrophic nature of fungi4. The obligate symbiotic nature of AM fungi is a crucial factor in the mass multiplication of AM spores in vitro. The existing methods of AM fungal cultivation are pot culture technique, aeroponics and root organ culture. Microbial contamination is the drawback of these methods. So the best strategy is to co-cultivate AM fungi with Ri T-DNA transformed roots.
Transformed roots were induced by wounding and infecting the host plants with A. rhizogenes. The rhizogenicity is conferred to plant cells by a fragment of DNA (Ri T-DNA), which is transferred from the large root-inducting (Ri) plasmid, harbored by the bacterium, to the genome, where it is stably integrated and expressed. Integration of a DNA segment (T-DNA) of pRi into the host genome leads to active proliferation of adventitious roots (hairy roots) at or near the site of infection5 and produce growth hormones in them.
The Ri T-DNA transformed roots are used for a wide range of fundamental and applied studies. One of the most important of these has been the study of the AM symbiosis6 and successful colonization of Glomus mosseae spores in Convolvulus sepium hairy roots for the first time. Mosse and Hepper7 developed the first simple in vitro systems for culturing AMF and these crude systems have since been refined to current monoxenic, hydroponic and aeroponic systems. Numerous authors6,8,9,10 used Ri T-DNA transformed hairy roots of carrot or tomato for cultivating mycorrhizal fungi. The use of transformed carrot roots, in place of whole plants, provided a simple in vitro system, which allowed reproducible observation of all stages of vesicular-arbuscular mycorrhizal (VAM) development under aseptic conditions11. The combination of transformed carrot roots and sterile AMF spores can be used to produce “dual in vitro cultures” that provide an efficient method of producing abundant (typically over 5000) spores and mycelia in a nine centimeter petri plate8,12 .The monoxenic method involves growing sterile AMF spores on dual culture plates with transformed carrot (Daucus carota) roots13. Potty and Chandran14 successfully co-cultivated G. microcarpum in Ri T-DNA transformed cassava roots.
The objective of this study was to successfully co-cultivate G. microcarpum in transgenic hairy roots of I. batatas (variety S1010) initiated through the mediation of Agrobacterium rhizogenes ATCC 15834 and to study the various stages of mycorrhizal infection and its viability. Most of the authors so far used carrot hairy roots because of its vigour and ease with which it can be propagated. In the present investigation, attempts were made to colonize mycorrhizal spores of G. microcarpum in hairy roots of I. batatas variety S 1010 and this is the first report. This technique can also be used for the large scale production of monospecific AM fungal inoculum.
Materials and Methods
Sterilization and germination of seeds
The seeds of I. batatas (variety S1010) were surface sterilized primarily with mild detergent solution followed by treatment with 0.1% mercuric chloride solution for 10 minutes and the seeds were washed well with distilled water. Second stage sterilization was done with 70% ethanol for 3 minutes and washed well with distilled water. The surface sterilized seeds were placed in 1% sterilized agar plates and kept at room temperature for incubation.
Hairy root induction in I. batatas
rhizogenes ATCC 15834 was used to induce hairy root infection and cultured in yeast extract medium15 which contained beef extract – 5g, yeast extract – 1g, peptone – 5g, sucrose – 5g, MgSO4 7H2O – 0.49g, bacto agar – 12g, distilled water – 1000 ml and pH – 7.2. Bacterial culture was incubated at 24oC in the dark for 48 hours. In vitro seedlings of I. batatas (variety S1010) were wounded on the stem using a sterile scalpel, which was smeared with 48 h old culture of A. rhizogenes and incubated at 24oC for two days in dark. After two days of incubation the explants were transferred to fresh MS medium, containing cefataxime 250 mg/l to eliminate excess bacterial growth. Then the explants were transferred to fresh Murashige and Skoog16 medium without antibiotic and kept for further incubation for hairy root emergence17. Hairy roots emerged were cut approximately 2 centimeter long and were transferred to standardized modified MS medium and incubated at 24oC.
Isolation and surface sterilization of mycorrhizal spores
AM fungal spores were isolated from pot culture maintained in glass house condition in Central Tuber Crops Research Institute, Thiruvananthapuram, Kerala State, India, by wet sieving and decanting method18. Mycorrhizal spores of Glomus microcarpum were surface sterilized according to Mertz et al.19. The sterilized spores were kept in a solution containing 2% Chloramine T and 1-2 drops of Tween 20 for 10 minutes and rinsed them with sterile distilled water and repeated treatment with Chloramine T for 10 minutes and rinsed them with sterile distilled water. The spores were transferred to sterile solution containing 200 mg/l streptomycin and 100 mg/l gentamycin and stored the solution at 4oC.
Mycorrhizal infection
Standardized modified Murashige and Skoog (MS) medium was prepared and poured in 9 cm petri plates. Fresh transformed roots approximately 3 to 4 centimeters long were placed in the medium. In order to obtain dual culture of AM fungus in transformed roots, fungal inoculum, the surface sterilized spores were placed near the transformed roots at a distance of 5 millimeter and kept for incubation at 28oC. Different stages of mycorrhizal infection such as pre-infection, penetration, vesicle/arbuscule formation and colonization were studied.
Viability of mycorrhizal fungi
Activity of succinate dehydrogenase (SDH), a mitochondrial enzyme was considered as an indicator of viability of mycorrhizal fungi20. The transformed root samples were cut approximately to 1 cm and were washed with ice-cold water and kept in ice. Weighed 0.2 to 0.5 g of root samples in a test tube and added 20 ml 0.05 M Tris/ HCl pH 7.4, 50 mg/ml sorbitol, 15 units/ml cellulase (from Aspergillus niger) and 15 units/ml pectinase (from A. niger) to the root samples and kept it for 2 hours at room temperature for clearing. Clearly rinsed the roots with water on a fine sieve. Transferred the root samples to a bottle and added 20 ml of SDH staining solution [Tris/HCl, pH 7.4 (0.2 mol.l-1) 2 ml, MgCl2 (5 mmol.l-1) 2 ml, Nitro-blue Tetrazonium (4 mg ml-1) 5 ml, Na-succinate (2.5 mol.l-1) 6 ml and H2O 2ml] and incubated the root pieces overnight at room temperature. After incubation poured out the staining solution and thoroughly washed with distilled water. Treated the roots with sodium hypochlorite solution (containing 3% active chlorine) for 5 minutes and washed with distilled water. Transferred the root bits to petri dish and observed the stained mycorrhizal hairy roots under the microscope.
Results and Discussion
Transgenic hairy roots emerged from the wounded sites of in vitro plants of I. batatas variety S1010 (Fig. 1) on the 10th day and the hairy roots were initially white and turned slightly yellow. They were finely branched and exhibited negative geotropism. The roots were successfully grown in modified MS medium, in which the MS salt concentration was reduced to 1/5 strength. Full and 1/2 strength MS concentrations resulted in the browning of hairy root tissues. So further dilutions were made and 1/5 strength was found to be the optimum concentration for hairy root growth. MS vitamins were replaced with B5 vitamins21 and sucrose concentration was reduced from 30g/l to 15g/l. Cystine HCl was used as an antioxidant which prevented the browning of plant tissues. The standardized medium composition and other growth parameters are given in Table I.
The hairy roots emerged from the wounded sites were successfully transferred to standardized modified MS medium containing cefataxime 250 mg/l to contain residual Agrobacterium growth. Prolonged exposure of plant tissues to cefataxime leads to the death of tissues and two to three successive transfers were made to free the hairy roots from Agrobacterium. The bacteria free hairy roots were used to infect mycorrhizal fungi.
Table I. Standardized medium and growth conditions
|
Medium Composition
|
Temperature |
pH |
Light |
|
|
MS salts
B5 vitamins
Myoinositol Nicotinic acid Pyridoxine HCl Thiamine HCl
Sucrose
Cysteine HCl
Agar
Distilled water |
1/5 strength
Full strength
100 mg/l 1.0 mg/l 1.0 mg/l 10 mg/l
15 g/l
0.5 g/l
7 g/l
1000 ml |
24oC |
5.7- 6 |
16 hours photo period
|
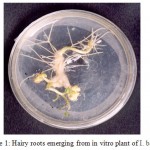 |
Figure 1. Hairy roots emerging from in vitro plant of I. batatas.
|
The surface sterilized spores served as infective propagules and the fungal development initiated from them. Surface sterilized spores of G. microcarpum placed near the hairy roots germinated and infected the hairy roots. After sterilization, germination percentage of spores was 95 % and they germinated within 2 to 4 days. Five percent of the spores failed to germinate and this may be either due to the strong sterilization treatment or the spores were immature, dormant, or non viable.
The growth medium, 1/5 strength of MS medium used for supporting hairy roots did not affect the mycorrhizal (Glomus microcarpum) infection in hairy roots. The higher concentration of P and N in MS medium was found to be inhibitory for AM fungal colonization22. The pH 5.7 to 6 was found to be the optimum for the growth of I. batatas hairy roots in modified MS medium and this pH range also supported the establishment of mycorrhizal fungi in hairy roots even though a neutral to alkaline pH was said to be optimum to Glomus species23. Green et al.24 also reported that Glomus spp. germinated between pH 6 and 9. These observations varied slightly with the present investigation.
Optimal temperature for spore germination seemed to vary among AM fungal species. The optimum temperature used for I. batatas hairy root proliferation was 24oC and this was also found optimum for the germination of G. microcarpum spores where as, Glomus mosseae and Acaulospora leavis germinated between 10 to 18 and 30oC with an optimum between 20 and 25oC. Gigaspora germinates between 10 and 30oC with an optimum between 20 and 30oC25 and germination was best at 10 to 25oC for Glomus caledonium26 and 25oC for Glomus epigaeum27.
Different stages such as pre-penetration, penetration, vesicle/ arbuscule formation was observed (Table II). In the primary infection, fungus penetrated the roots between the epidermal cells and forms appressorium in the first cell layers. The pre-penetration stage was observed on 6th day of infection. Vesicle/arbuscules were found on the mycorrhiza infected hairy roots of I. batatas from the 10th day onwards (Fig. 2, 3) and this clearly indicated the successful mycorrhizal colonization in Ri T-DNA transformed I. batatas hairy roots. After 20 days of co-cultivation growth, the hairy roots were subjected to SDH analysis and confirmed the viability of AM fungi. The stained root bits showed a dark purple spots indicated SDH activity (Fig. 4). This enzyme was chosen because SDH activity is a marker for respiratory activity.
Table 2. Stages of mycorrhizal infection in hairy roots.
| Days
Stages
|
2 |
4 |
6 |
8 |
10 |
12 |
14 |
16 |
18 |
20 |
|
Pre infection
|
– |
– |
+ + |
+ + |
+ + |
+ + + |
+ + + |
+ + + + |
+ + + + |
+ + + + +
|
|
Primary infection (Root penetration)
|
– |
– |
– |
+ + |
+ + |
+ + + |
+ + + + |
+ + + + |
+ + + + |
+ + + +
|
| Vesicles and arbuscule formation
|
–
|
– |
– |
– |
+
|
+
|
+
|
+
|
+
|
+
|
|
Colonization
|
– |
– |
– |
++ |
+ + + |
+ + + + |
+ + + + |
+ + + + |
+ + + + |
+ + + +
|
“Each + represents 20% colonization”
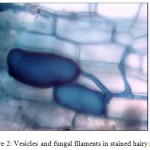 |
Figure2. Vesicles and fungal filaments in stained hairy roots.
|
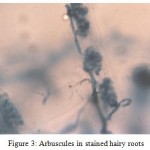 |
Figure 3: Arbuscules in stained hairy roots.
|
 |
Figure 4: SDH activity in mycorrhized hairy roots
|
During the pre-infection stage, the spores of G. microcarpum were kept near the hairy roots of I. batatas and infection was observed on 6th day and 40 percent colonization was found in the hairy roots. Penetration stage was found in the eighth day of infection with 40% mycorrhizal colonization.
During the course of this study 80% mycorrhizal colonization was achieved and also found that I. batatas was highly susceptible for mycorrhizal colonization, where as Abdul-khaliq and Bagyaraj28, 2000, colonized Gigaspora margarita in tomato hairy roots and reported 30% mycorrhizal colonization after 30 days. The higher mycorrhizal colonization percentage in I. batatas hairy roots was attributed to the higher dependency of mycorrhizal fungi towards I. batatas variety S 1010. The successful colonization of AM fungi Glomus microcarpum in transgenic hairy roots of I. batatas is for the first time. This variety is also resistant to root knot nematode and this fungi is also known to inhibit the growth and establishment of root knot nematode29. Diop30 also reported that the AM fungus increased protection of the root by reducing the nematode population by 50%. I. batatas variety S 1010 showed a higher colonization percentage more than 80% in normal roots in field conditions and prevents the entry of nematodes. This variety is also used as a trap crop for nematodes and because of this reason this variety was chosen for this study. This mycorrhized inoculum can be recommended to field crops to reduce root knot nematode incidence. These mycorrhized hairy roots can also be grown in bioreactor using liquid medium for the large scale production of axenic mycorrhizal inoculum.
References
- Smith S. A. and Read D., Mycorrhizal Symbiosis, 2nd Edn., Academic Press, Inc., San Diego (1997).
- Sylvia D. M., Rhizosphere Dynamics, Westview Press, Boulder, 145-167 (1990).
- Jakobsen I., Abbott L. K. and Robson A. D., New Phytol., 120, 509-516 (1992).
- Williams P. J., Method Microbiol., 24, 203-220 (1992).
- Chilton M. D., Tepfer D. A., Petit A., David C., Casse-Delbart F. and Tempe J., Nature, 295, 432-434 (1982).
- Mugnier J. and Mosse B., Phytopathology., 77, 1045-1050 (1987).
- Mosse B. and Hepper C., Physiol. Plant Pathol., 5, 215-223 (1975).
- Becard G. and Fortin J. A., New Phytol., 108, 211-218 (1988).
- Declerck S., Strullu D.G. and Plenchette C., Mycol. Res., 100, 1237-1242 (1996).
- Jolicoeur M., Williams R. D., Chavarie C., Fortin J. A. and Archambault J., Biotechnol. Bioeng., 63, 224-232 (1999).
- Chabot S., Becard G. and Piche Y., Mycologia., 84, 315-321 (1992).
- Diop T. A., Becard G. and Piche Y., Symbiosis., 12,249-259 (1992).
- St-Arnaud M., Hamel C., Vimard B., Caron M. and Fortin J. A., Mycol. Res., 100, 328-332 (1996).
- Potty V. P. and Chandran R. P., Proceedings of 5th international meeting of the Cassava Biotechnology Network, St. Louis Mo, USA (2001).
- Vervliet G., Holsters M., Teuclay H., Van Montagu M. and Schell J., J. Gen. Virol., 26, 33-48 (1974).
- Murashige T. and Skoog F., Plant Physiol., 15, 473-473 (1962).
- Ooms G., Karp A., Burell M. M., Twell D. and Roberts., Theor. Appl. Genet., 70, 440-446 (1985).
- Gerdemann J. W. and Nicolson T.H., Trans. Br. Mycol. Soc., 46, 235-244 (1963).
- Mertz Jr. S. M., Heithas III J. J. and Bush R. L., Trans. Br. Mycol. Soc., 72,167-169 (1979).
- Vierheilig H. and Ocampo J. A., Agricult. Ecosys. Environ., 29, 439-442 (1989).
- Gamborg O. L., Miller R. A. and Ojima K. Exp. Cel. Res., 50, 155-158 (1968).
- Bressan, W. Braz. J. Microbiol. 33, 33-34 (2002).
- Siqueira J. O., Hubbell D.H. and Mahmud A.W., Plant and Soil, 76, 115-124 (1984).
- Green N. E., Graham S. O. and Schenck N. C., Mycologia., 68, 929-934. (1976).
- Safir G. R., VA Mycorrhizae: an ecophysiological approach, Safir GR (ed) Ecophysiology of VA mycorrhizal plants, CRC Press, Boca Raton, 1-3 (1986).
- TommerupI. C., Trans. Br. Mycol. Soc., 81, 381-384 (1983).
- Graham J. H., Mycologia., 74, 831-835 (1982).
- Abdul-Khaliq and Bagyaraj D. J., Indian J. Exp. Biol., 38, 1147-1151 (2000).
- Mohandas C. and Ramakrishnan S., J. Root Crops., 22, 135-136 (1996).
- Diop T. A., Afr. J. of Biotechnol., 2, 692-697 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.





