Manuscript accepted on : January 04, 2009
Published online on: --
Hepatoprotective Activity of Colocasia Antiquorum Against Alcohol Induced Liver Injury In Rats
T. A. Tuse, U. N. Harle*, N.S.Vyawahare, V. V. Bore and P. R. Gangurde
Department of Pharmacology, AISSMS College of Pharmacy, Near R.T.O., Kennedy Road, Pune - 411 005 India.
Corresponding Author E-mail: unharle@yahoo.co.in
ABSTRACT: Colocasia antiquorum (L.) Schott, (Araceae), is an ancient crop grown throughout the humid tropics. The aim of this work was to study the hepatoprotective effect of crude ethanolic extract of corms of C. antiquorum using alcohol induced hepatotoxicity in rats as experimental models. The chronic treatment of extract of C. antiquorum at a dose of 500 mg/kg was administered orally for 21 days. The protective effect was evident from serum biochemical parameters and histopathological analysis. Rats treated with extract of C. antiquorum significantaly prevented the elevation of SGPT, SGOT, SALP, Cholesterol and Triglyceride in alcohol treated rats. Histopathological changes induced by alcohol were also significantly reduced by the extract treatment. The activity of ethanolic extract of C. antiquorum was nearly similar to that of silymarin, the reference hepatoprotective drug. Further it shows that the corms of C. antiquorum contains anthocyanins such as cyanidin 3-glucoside, pelargonidin 3-glucoside and cyanidin 3-rhamnoside, may be responsible for the hepatoprotective activity. Thus it may conclude that ethanolic extract of C. antiquorum possesses significant hepatoprotective properties.
KEYWORDS: Alcohol; Colocasia antiquorum; silymarin; ethanolic extract; anthocyanins
Download this article as:| Copy the following to cite this article: Tuse T. A, Harle U. N, Vyawahare N. s, Bore V. V, Gangurde P. R. Hepatoprotective Activity of Colocasia Antiquorum Against Alcohol Induced Liver Injury In Rats. Biosci Biotechnol Res Asia 2009;6(1).. |
| Copy the following to cite this URL: Tuse T. A, Harle U. N, Vyawahare N. s, Bore V. V, Gangurde P. R. Hepatoprotective Activity of Colocasia Antiquorum Against Alcohol Induced Liver Injury In Rats. Biosci Biotechnol Res Asia 2009;6(1).. Available from: https://www.biotech-asia.org/?p=20105 |
Introduction
C. antiquorum (L.) Schott, (Syn. Colocasia esculenta, Family; Araceae) commonly known as ‘Taro’, which is an ancient crop grown throughout the humid tropics for its edible corms and leaves, as well as for its traditional uses 1. The corms of C. antiquorum contain the anthocyanins as cyanidin 3-glucoside, pelargonidin 3-glucoside and cyanidin 3-rhamnoside, reported to have antioxidant and anti-inflammatory properties 2. It also has the anti-cancer activity on colonic adenocarcinoma cells 3. Extract containing cyanoglucoside of C. antiquorum possesses hypoglycemic property 4. Digalactosyl and monogalactocyl diacylglycerols, which are identified as anti-hyperlipemia active components in C. antiquorum 5. Cystatin from C. antiquorum have antifungal activity 6. An arabinogalactan from C. antiquorum tubers have hypolipidaemic effect 7. Tubers of C. antiquorum have amylase, trypsin and chymotrypsin inhibitory activities 8.
Traditionally C. antiquorum is used to settle the stomach, to prevent swelling and pain; and to reduce fever. It is also used as a poultice on infected sores 9. C. antiquorum is used in folklore as a medicine for liver complaints. Therefore present study was undertaken to scientifically prove the folklore use of the plant against hepatotoxicity.
Materials and Methods
Plant material
antiquorum L. (Schott.) was collected from local areas near Pune, India and authenticated by the Botanical Survey of India, Regional centre, Pune. Voucher specimen (BSI/WC/Tech./2008/1086) was deposited in the Herbarium of Botanical Survey of India, Regional centre, Pune.
Preparation of extracts
The shade dried corms of about 1 kg were subjected for size reduction to coarse powder. The ethanol extract was prepared by maceration process for 7 days following defatting by pet ether. The solvent was removed completely under reduced pressure. Yield of the extract was 2.5 % (w/w). The ethanolic extract was found to contain anthocyanins as cyanidin 3-glucoside, pelargonidin 3-glucoside and cyanidin 3-rhamnoside.
Animals
Wistar albino rats of either sex, weighing 200-250 g and Swiss Albino mice of either sex, weighing 22-25 g, were maintained under standard laboratory conditions (temperature 23 ± 2 0C, relative humidity 55 ± 10 %) were used for experiments. Animals were allowed to take standard laboratory feed and water. The experiments were conducted according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India and approved by Institutional Animal Ethical Committee (approval no. CPCSEA/IAEC/PC-08/09-2K7).
Drugs and chemicals
Alcohol (food grade) procured from Cosmochem, Pune and silymarin procured from Sanjivani Herbals, Mumbai.
Behavioral effect and toxicity
Albino mice (Swiss strain) divided into five groups of five animals each and treated with graded dose (200, 400, 600, 800, and 1000 mg/kg; p.o., respectively) of the extract. The mice were observed continuously for 1 hour for any gross behavioral changes and death if any, intermittently for the next 6 h, and then again at 24 h after dosing.
Alcohol induced hepatotoxicity
Twenty rats of either sex distributed into four groups of 5 animals each. Rats of each group were treated for 21 days. Group-I which served as control in addition to normal diet received 1% Gum acacia. Group- II in addition to normal diet received 40% alcohol solution(v/v, 2ml/ 100g bw; p.o.). Group-III in addition to normal diet received 100 mg/kg silymarin 30 min. before the administration of 40% alcohol solution(v/v, 2ml/100g, bw; p.o.). Group-IV in addition to normal diet received 500 mg/kg extract of C. antiquorum 30min. before the administration of 40% alcohol solution (v/v, 2ml/ 100g, bw; p.o.). The serum was isolated and used for the assay of the marker enzymes as SGPT, SGOT, SALP, Cholesterol and Triglyceride.
Assessment of liver function
Blood samples of the rats were withdrawn from ratino bulber venous plexus with the help of a glass capillary under light ether anesthesia and were kept at room temperature for 2 hr, so that the process of coagulation gets completed. The blood samples were centrifuged and serum thus separated which was used for the estimation of SGPT, SGOT, SALP, Cholesterol and Triglyceride. Animals were then sacrificed and dissected. Their livers were taken out, washed with water, dried gently with filter paper and preserved in 10% formolsaline. The SGPT, SGOT, SALP, Cholesterol and Triglyceride were determined with a commercial kit by autoanalyser of Merck make (300 TX, E. Merck-Micro Labs, Mumbai).
Histopathological examination of the liver
The livers of the experimental animals were fixed in 10% formol-saline prior to routine processing in paraffin-embedded blocks. Sections (5μm thick) cut and stained using hematoxylin–eosin (HE) stain. Sections observed under microscope and photomicrographs were taken.
Statistical Analysis
Results were expressed as Mean ± S.E.M. The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey test and p<0.05 considered as statistical significant.
Results
Preliminary phytochemical screening of C. antiquorum
Preliminary phytochemical screening indicated the presence of anthocyanins such as cyanidin 3-glucoside, pelargonidin 3-glucoside and cyanidin 3-rhamnoside.
Behavioral effect and toxicity
The extract of C. antiquorum was found to be safe for further biological studies, as no lethality were observed even at 1000 mg/kg, orally in mice.
Alcohol induced hepatotoxicity
Alcohol administration resulted in a significant elevation of AST, ALT, SALP, triglyceride and cholesterol to be decreased as compared to normal control group. The ethanolic extract of C. antiquorum shows significant (p<0.001) decrease in the levels of SGPT, SGOT, SALP, Cholesterol and Triglyceride (fig.1). Pretreatment with silymarin significantly prevented the biochemical changes induced by alcohol.
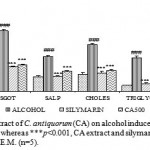 |
Figure 1: Effect of ethanolic extract of C. antiquorum (CA) on alcohol induced hepatotoxicity in rats. ### p<0.001, alcohol vs. control, whereas ***p<0.001, CA extract and silymarin vs. paracetamol. Values are considered as mean ± S.E.M. (n=5).
|
Histopathology of alcohol induced hepatotoxicity
The liver of the control animal (fig.2A) showed the normal lobular architecture. The lobulation is modest as a result of low content of interstitial tissues, and can be determined only with reference to the central vein. The hepatocytes are arranged in a series of brancing and anatosmosing perforated laminae to form a labyrinth, between sinusoidal spaces.
The liver section treated with 40% alcohol (fig. 2B). Where, it was observed that the hepatocytes were swoolen and the sinusoidal spaces occluded. The normal lobular architecture pattern of the liver section can not be discerned, though the central vein is seen. The liver section treated with ethanolic extract of C. antiquorum (fig.2C) showed a histopathological picture that closely approximates that of the control group. The plate like arrangement of the hepatocytes were seen, the sinusoidal spaces were also visible, but not as prominent as in control group. The liver section treated with standard drug silymarin (fig.2D) showed the normal hepatic architecture as compared to control group. The arrangement of hepatocytes was same as to control group.
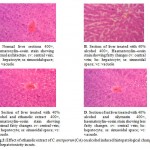 |
Figure 2: Effect of ethanolic extract of C. antiquorum (CA) on alcohol induced histopatological changes of hepatotoxicity in rats.
|
Discussion
In Indian system of medicine and classical literature of C. antiquorum is claimed to provide relief against liver disorders and have to folklore. C. antiquorum was evaluated for effect on alcohol induced hepatotoxicity. The ethanolic extract of C. antiquorum showed significant hepatoprotective effect in the alcohol intoxicated rats. Corms of C. antiquorum contain anthocyanins reported to have antioxidant activity however, anthocyanins such as delphinidin, cyanidin, and pelargonidin 10, 11, which may be responsible to increase the resistance of hepatocytes in alcohol induced oxidation.
The hepatocytes contain three main pathways for alcohol metabolism to give acetaldehyde, each located in a different sub cellular compartment, namely the alcohol dehydrogenase pathway of cytosol, the microsomal alcohol oxidizing system located in the endoplasmic reticulum, and catalase located in the peroxisomes. Chronic alcohol feeding results in appearance of a form of cytochrome P-450 also differing by its catalytic activity from other cytochrome P-450 species12. The effect of C. antiquorum on AST, ALT, SALP, triglyceride and cholesterol are of great importance in understanding the effects of alcohol on liver.
From this investigation it can be concluded that the hepatoprotective effect of ethanolic extract of corms of C. antiquorum was observed in alcohol intoxication in rats. The potency of C. antiquorum was compared with silymarin showing good activity on hepatic markers with respect to this reference compound.
Acknowledgement
The authors are grateful to the Principal Prof. K.G.Bothara AISSMS College of pharmacy, Pune for providing necessary support.
References
- Miller C. D. Food values of Poi, Taro, and Limu. Bernice P., Bishop Museum Bulletin, 37, Honolulu, Hawaii, 235 (1971).
- Cambie R. C. and Ferguson L. R., Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 524, 109-117 (2003).
- Brown A. C., Reitzenstein J. E., Liu J. and Jadus M. R., In vitro. Phytotherapy Research, 9, 767-771 (2005).
- Phillip B. A., Grindleya O. F., Asemota H. N., Errol Y. and Morrisona A., Nutrition Research, 22, 333-341 (2002).
- Tanaka R., Sakano Y., Nagatsu A., Shibuya M., Ebizukab Y. and Goda Y., Bioorganic & Medicinal Chemistry Letters, 15, 159-162 (2005).
- Yang A. H. and Yeh K. W., Planta, 221:4, 493-501 (2005).
- Boban P. T., Nambisan B. and Sudhakaran P., British Journal of Nutrition, 6, 1021- 1028 (2006).
- Prathibha S., Nambisan B. and Leelamma S., Journal of food processing and preservation, 22, 359-370 (1998).
- Nair R., Kalariya T. and Chanda S., .Turk Journal of biol, 29, 41-47 (2005).
- Kowalczyk E., Kopff A. and Fijalkowski P., Acta Biochem Pol., 50, 543-548 (2003).
- Noda Y., Kaneyuki T. and Mori A., Journal of Agric Food Chem, 50, 166-171 (2002).
- Giljoly J., Villeneuve J. and Mavier P., Biochemical Pharmacology, 55, 34-41 (1977).

This work is licensed under a Creative Commons Attribution 4.0 International License.





