Manuscript accepted on : 29-07-2020
Published online on: 08-09-2020
Plagiarism Check: Yes
Reviewed by: Ayan Mukherjee
Second Review by: Biswajit Batabyal
Final Approval by: Prof. Dr.Majid Sakhi Jabir ![]()
![]()
Dengue Vector Control: A Review for Wolbachia-Based Strategies
Mohammed A. Alkuriji1, Mohamed B. Al-Fageeh2, Fekri M. Shaher1* and Bassam F. Almutairi1
and Bassam F. Almutairi1
1National Center of Agricultural Technology, Life Science and Environmental Research Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia
2Director-General, General Directorate for Research and Innovation Support (GDRIS) King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia
Corresponding Author E-mail : fikry1978@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2854
ABSTRACT: Mosquito-borne diseases continue to pose a major health problem globally and have had a significant impact on human life and economy. Consequently, many countries have implemented national vector control programs in an effort to suppress/eradicate mosquitos contributing to spread of diseases including Malaria, Dengue, Yellow fever, Rift valley fever, West Nile fever, Zika, Chikungunya etc. Of these endemic diseases, Dengue fever is an arbovirus and transmitted primarily by Aedes aegypti mosquito that has become a rapidly emerging infection, especially in the tropical countries. Insecticides spraying remains the main method to control the transmition of dengue virus. However, the overuse and misuse of insecticides can result in negative consequences such as the development of insecticides resistance. This, in part, has led to the development of a more eco-friendly measures to suppress mosquitoes e.g. gene-drive based controls and Wolbachia-based approaches. The latter approach has the ability to block the dengue virus transmission by inhibiting virus intracellular replication in mosquito. In addition, Wolbachia decreases adult mosquito lifespan and can be naturally passed from one generation to the next. In recent years, Aedes aegypti mosquitos infected with Wolbachia released and tested in the field in several countries and have achieved very promising results. In this review, we focus and discuss the emerging Wolbachia-based biocontrol approaches that are already being deployed, evaluated and tested in the field.
KEYWORDS: Aedes aegypti, Dengue, Wolbachia, Cytoplasmic incompatibility (CI)
Download this article as:| Copy the following to cite this article: Alkuriji M. A, Al-Fageeh M. B, Shaher F. M, Almutairi B. F. Dengue Vector Control: A Review for Wolbachia-Based Strategies. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Alkuriji M. A, Al-Fageeh M. B, Shaher F. M, Almutairi B. F. Dengue Vector Control: A Review for Wolbachia-Based Strategies. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/35hq42W |
Introduction
Global incidences of mosquito-borne diseases are growing up due to people travel, fast urbanization and ineffective of control programs measures.1 Dengue fever (DENV) is the most serious arboviral epidemic threatening humanity and it is responsible of death cases in the tropical and subtropical regions. About 50 % of the inhabitants across the world are now at dengue risk. Official reports estimated around 390 million person are infected annually, half million of them are critical situations and require hospital treatment. About of 2.5 % of those infected cases die. Although several countries used vaccine against DENV in humans ranged between 9 and 45 years of age inhabiting in endemic areas, but the mosquito control is still the main approach to stop the dengue disease.2 A. aegypti mosquito is the main vector of Dengue, Zika, chikungunya and yellow fever diseases, also A. albopictus is a possible transmitter. A. aegypti mosquito is well adapted to mankind. The mosquito females obtain blood meals by biting and digestive it inside their bodies to produce their eggs. Unfortunately, the elimination of A. aegypti is not easy task due to their ability to lay the eggs in many sites included those of a little amount of water. Also the eggs able to stay alive months in the absence of water and hatch as soon as water is available. Moreover they have resistance to common insecticides.3,4 All these conditions make the eradication of A. aegypti by traditional techniques useless.5 The use of insecticides can be effective on mosquito control program, but it is often prohibitively, highly cost, harmful on non-target organisms, has negative environmentally effects. Furthermore, the long term use of insecticides led mosquitoes to develop resistance against insecticides.6,7 Alternative methods were used to mosquitoes management such as the elimination of eggs laying sites, the using of animals that naturally preys on mosquitoes like copepods and fish8 and avoid mosquito bites by protection tools. These strategies are considered beneficial way in some cases but it can be difficult and high cost to apply in urban areas. Accordingly, Novel arbovirus vector control tools are needed. Currently, two novel techniques revealed promise in reducing the dengue transmission.9 The first one depends on is a genetic management by spread mosquitoes that are treated with lethal or flightless trait10 and the second technique is establishment of mosquitoes carrying Wolbachia bacterium. Wolbachia block and prevent the growth of the dengue virus inside A. agypti mosquitoes.11,12 In this technique, the Wolbachia-infected mosquitoes are released into the wild. Because of cytoplasmic incompatibility (CI), the wolbachia are passed on through generations of mosquitoes and the ratio of Wolbachia-infected mosquitoes is growing up until it become high and predominance without any additional releases. This strategy is applied in several countries.13,14,15
What is Wolbachia?
Wolbachia is considered type of Gram-negative bacterium fall under the order Rickettsiales and the family Anaplasmataceaet (Table 1). This type of bacterium are naturally available in invertebrates and infect about 60% of insect community.11 However, Wolbachia is naturally absent from A. aegypti mosquito, the main transmitter responsible for the spread of human diseases including dengue and other diseases of RNA-virus. Wolbachia has the ability to prevent the growth of several of RNA-viruses in mosquitoes and Drosophila. If infected or uninfected males fertilize wolbachia-possitive females, the resulting generations will be healthy and carrying Wolbachia and are expanded in the wild population. Otherwise if infected males fertilize uninfected females, the resulting offspring could not developed. This event is named by the term of ̋cytoplasmic incompatibility̏ (CI). Meanwhile, Wolbachia-positive mosquitoes produce less eggs and reduce mosquito lifespan.
In general, the Wolbachia species are named based on the source where they first discovered. For example, Wolbachia pipientis (wPip) strain was isolated for the first time from Culex pipiens mosquito. Also, wMel species from the fruit fly Drosophila melanogaster, while wAlb species isolated from the mosquito Aedes albopictus. Scientists have revealed that Wolbachia stimulate the resistance of arthropods against viruses and inhibit their reproductive ability inside the host. Recently, Australian researchers of the control program showed of dengue have showed that the expand of wolbachia into wild mosquitoes A. aegypti populations is considered promising technique to overcome the dengue virus transmission. This led WHO and health authorities to encourage use Wolbachia approach as a way to overcome the transmission of dengue and arboviral diseases.12
Table 1: Taxonomy of Wolbachia and Aedes Aegypti
| Name | Taxon | Name | Taxon |
| Aedes Aegypti | Wolbachia | ||
| Animalia | kingdom | Bacteria | Domain |
| Arthropoda | phylum | Proteobacteria | Phylum |
| Insecta | Class | Alphaproteobacteria | Class |
| Diptera | Order | Rickettsidae | Subclass |
| Culicoidae | Superfamily | Rickettsiales | Order |
| Culicidae Meigen, 1818 | Family | Rickettsiaceae | Family |
| Culex, Aedes, Anopheles, etc | Genus (112) | Wolbachia | Genus |
| Aedes aegypti Linnaeus, 1762 | Species | Wolbachia pipientis, Hertig 1936 | Species |
Wolbachia strategy provides eco-friendly and a safe alternative to insecticide use. Although Wolbachia-infected A. aegypti were originally developed for biocontrol of dengue, it may able to reduce the transmission of other mosquito-borne diseases including chikungunya and yellow fever,16 potentially malaria17,18 and Zika.19
Potential Risk of Wolbachia-Infected Mosquitoes on Human
There is no evidence indicate that wolbachia transfer to human or to the mosquito predators such as geckos and spiders. No antigenic or immune response developed by mosquitoes bites.20 The Australian Commonwealth Scientific Organization produced a risk assessment of releasing Wolbachia in the wild [21] before the official authorities granted the acceptance.13
It is worth noting that the Wolbachia does not horizontally transfer to other organisms. Potentially, there is horizontally transfer of Wolbachia DNA into mosquito genomes, but this situation of transfer happen rarely.22,23,24,25 Such lateral transfer are unlikely to raise the risk related with the Wolbachia-positive mosquitoes release. Wolbachia-based biocontrol holds the promise of an environmentally and safe alternative that is not expensive to implement and has the chance to be effective on a global scale.
Biocontrol of Dengue Virus Using Wolbachia Strategy
Recently, the wolbachia has been studied by several researchers for its potential to use as a biocontrol strategy of Aedes mosquito.26 Laven (1967) was the first researcher started the use of Wolbachia-infected Cx. pipiens mosquitoes to eliminate the the population of mosquito Culex pipiens through cytoplasmic incompatability (CI).27 CI is a phenomenon occurs when wolbachia-infected males are mating with uninfected females and the resulting offspring can not develop. In contrast, when both Wolbachia-positive male and female are mating, the offspring will hatch and develop normally.28
Wolachia release was done in Yorkeys Knob and Gordonvale, Australia, in early January 2011, as first trial sites, both wMel-positive females and males of A. aegypti were weekly released for a totally of ten weeks.
After five weeks-post finishing release, A. aegypti mosquitoes were Wolbachia positive with the percentage of 100 % and 90 % in Yorkeys Knob and Gordonvale, respectively.
Second release was in January 2012 by wMelPop-infected A. aegypti in both Machans Beach and Babinda areas. A promising proportion of wMelPop-positive A. aegypti was reported (with 49% and 75%, respectively) in the wild population during 2–3 weeks after the release start. However, one month post-finishing release, the proportions of wMelPop-infected A. aegypti decreased to less than 50 and 71 % in both Machans Beach and Babinda, respectively. This is may be attributed to inability of that Wolbachia strain to keep themselves for a long time in the field.29
Releases of wAlbB-infected Ae. Aegypti mosquitoes were done in greater Kuala Lumpur, Malaysia, including 6 diverse sites with high dengue cases. The wolbachia strain was established successfully with very high population frequency at some sites and fluctuations at other sites which were supported by additional releases. Based on the monitoring of the situation and compared to control sites, decrease in human dengue cases was observed in the release sites. The wAlbB strain of Wolbachia offers a promising strategy as a tool for dengue control, especially in very hot weather.30
Durovni et al., 2019 described study for evaluating the impact of wide-scale Wolbachia releases on the control of dengue, chikungunya and Zika in Brazil. The study is in progress and the monitoring and data analysis will continue until 2023. In case of success, the experiment will be expanded nationally and regionally. Releases programs of mosquito carrying Wolbachia are implemented or still in progress in 8 countries, fortunately no record of dengue, chikungunya and zika cases in areas where wide spread of Wolbachia-infected mosquitoes are established.31
Adekunle et al., 2019 described a dynamic model adjusting for deficient vertically transmission and decline of Wolbachia infection. This model shows clearly that the disadvantages of CI could outweigh the advantages and the Wolbachia may be lost. They set the optimal release strategy that determines the ability of Wolbachia for invasion and also, they deduced locally and globally stability of the equilibrium points.32
Mathematical modelling represents a significant tool to understand the effect of factors in infectious diseases dynamics and help in making decisions regarding the implementation of control programs.33 These models simulate the invasion of Wolbachia-infected A. aegypti into wild mosquito populations. 34,35,36 CI represents important factor on the replacement between Wolbachia-uninfected and Wolbachia-infected mosquitoes populations.37 Ndii et al. described a model for the competition between both infected and infected mosquitoe populations and demonstrated the main factors that control on this competition.38 Xue et al. developed the same model as Ndii et al and sex type is incorporated into the model and demonstrated that successful establishment of infected populations need releasing high amount of mosquitoes carrying Wolbachia.39 Mathematical equations were used to develop model for the mosquito contests between wolbachia-positive and wolbachia-negative ones, Zheng et al. demonstrated that the succeeded alteration of wolbachia-negative mosquitoes by positive ones need a careful release strategy and Wolbachia strain play role in this task.36 Qu et al. developed a model of designed release methods and extend the model to include the idea that mosquito female mate once.40 The model by Li and Liu was designed and took in consideration the combined variables of birth-rate, mortality rate, wolbachia type and the amount of wolbachia-infected mosquitoes released.41
OReilly et al. used different models to evaluate the negative consequences of dengue in Indonesia. They expect that Wolbachia technique can avoid up to 75 % of disease consequences in the country. Area-wide interventions such as wolbachia can display an effective way to protect humans more than individually measures, such as vaccinations, in such huge population density.42 Finally, all above mentioned models support the approach of ability of Wolbachia-infected mosquitos to replace the uninfected ones in wild populations
Novel Wolbachia Strains in Anopheles Malaria Vectors from Sub-Saharan Africa
Malaria is mosquito-borne disease and transmit to human by some Anopheles mosquito species. Historically, anopheles genus has been considered Wolbachia-free but has recently discovered in 5 Anopheles species in west Africa, Anopheles coluzzii, Anopheles gambiae, Anopheles arabiensis, Anopheles moucheti and Anopheles species A. These novel strains of Wolbachia have possibility to establish Wolbachia-infected anopheles mosquitoes, which could be used for control strategies to overcome the plasmodium parasite responsible for malaria incidence [43].
Prevalence Dynamic of Wolbachia and Cytoplasmic Incompatibility (CI)
It is known that Wolbachia passed on from generation to the next generation by vertical transmission. Infected-males cause excitation of cytoplasmic incompatibility (CI) and modification of their sperm and lead to offspring death in early development stages (Figure 1A). However, in females case, Wolbachia make encoding that keep the offspring to stay alive and successful development allowing the bacterium to dominate over the populations (Figure A1) [44]. When Wolbachia spread with high rate then the reproductive advantage of infected females will be greatest. Otherwise, If Wolbachia is low, females rarely mate with Wolbachia-infected males, then low chance of compatibility with these males and Wolbachia could be lost from the population (Figure 1B). Similar case happens when infected females don’t carry Wolbachia to the whole offspring. Wolbachia release should be with high amount to avoid losing the infection after release stop.45,46,47 Releasing of Wolbachia-positive A. aegypti was implemented in 2011 in both isolated Yorkeys Knob and Gordonvale areas and the infection kept spread for 2 year-post release stop.46 Recently release was applied in Cairns area where the mosquitos’ migration may occur from the release areas to surrounding areas and vice-versa, this may lead to decrease of Wolbachia infection level and ultimately the loss of Wolbachia. However, the infection was developed well showing that Wolbachia approach can be implemented in a wide-scale.48
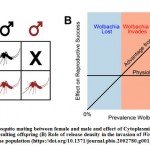 |
Figure 1: (A) Mosquito mating between female and male and effect of Cytoplasmic incompatibility (CI) on resulting offspring (B) Role of release density in the invasion of Wolbachia in the population |
Wolbachia and Pathogen Interference in Aedes aegypti
Creative strategy to control mosquito-born diseases started by using artificially Wolbachia-infected mosquitoes. Data from the field work has proved that Wolbachia represents promising technique to reduce natural populations of Aedes aegypti and control the diseases they transmit.
The mechanism of Wolbachia interference with the pathogens is complicated issue and need to be understood. Several scientists have attempted to explain the pathogen blocking by Wolbachia. They have discussed the properties of mosquito’s samples collected from the field and other related insects. They demonstrated the correlation between Wolbachia density and the ability to block pathogen by high load that destroy host tissue. Also the probability of induction the immune response system of the host which could resist the pathogens inside the insect. Furthermore, recent studies showed that Wolbachia play role in immune system modulation of the host and affect on the immunity system of A. aegypti and Culex quinquefasciatus to suppress Dengue and West Nile virus replication [49]. Other mode of action suggests modification of the cell membrane of the host, lead to preventing the vector to transmit the pathogens. Other explanation suggests competition development between Wolbachia and pathogens inside the host [50]. We mentioned above that wMelPop cause reducing in the lifespan of mosquito, this limits the pathogens spread because the lifespan of mosquito became shorter and not enough to complete the incubation interval for pathogens.51,52 Moreira et al. (2009) have described unusual behavior regarding the blood-feeding in wolbachia-infected A. aegypti, where proboscis becomes more prominent in elder mosquitoes, this phenomenon led to reduce the biting activities which eventually decrease the reproductive capacity.53 Additionally, Wolachia was found to enhance mosquito immune responses against pathogens. Bian et al. showed that the genes responsible for immune, Defensin, Cercropin, Diptericin, GNBPB1, SPZ1A, Cactus, Rel1 and Rel2 were adjusted in wolbachia-infected A. Aegypti mosquitoes, which could explain their ability to resist dengue virus.54
Wolbachia-Based Strategy to Control other Arboviral Infections
The technique of Wolbachia-infected mosquitoes was fucused initially for dengue control, experimental studies proved that this approach can extend to control other mosquito-borne diseases, particularly Chikungunya, Japanese encephalitis and Yellow fever. Regarding West Nile virus, it was recorded in 2009 that Wolbachia approach working to increase host resistance to West Nile virus in Culex quinquefasciatus mosquito.55 Subsequently, reports described that majority of Culex quinquefasciatus mosquitoes are naturally Wolbachia-infected but are still able to cause infection with West Nile virus. Furthermore, the Wolbachia strain isolated from Aedes albopictus play role in the enhancement of West Nile virus infection in Culex tarsalis, which is an important transmitter of West Nile virus in North America and naturally does carry Wolbachia.56 Finally, Wolbachia-infected mosquitoes showed high resistance to the transmission of two isolates of Brazilian Zika virus. Fortunately, no evidence that Wolbachia-infected A. aegypti is carrying Zika virus in the saliva, indicating that Wolbachia-based strategy can prevent the infection with of Zika virus.57
Wolbachia Strategy in Saudi Arabia
In the Kingdom of Saudi Arabia (KSA) dengue disease was recorded for the first time in 1994 [58] and the number of cases is growing up as reported by researchers, Malaria cases also represent issue especially in Jazan region. Although the control activities during the past period, but people remain at risk as the epidemics transmission does not stop. Thus, new control strategies are needed to overcome these health problems. Accordingly, the strategy of Wolbachia-based biocontrol of dengue is started and still in the initial stages. Outcomes will be subject to evaluation and reported after finishing releases. If the experiment achieved success, it could be implemented on a large-scale.
Conclusion
Mosquitoes transmit Dengue and several diseases. We discussed here the current available informations about the relation between Wolbachia and moaquitoes. Insecticide-based approaches are currently the key tools in combat of major mosquito-borne diseases. However, the ability of mosquitoes to develop resistance against insecticides in addition to its harmful effects to ecosystem push to thinking to find alternative strategies. Wolbachia is a promising as a bio-control technique in fighting mosquitoes-borne diseases. More research is urgently needed to find better understand about behavior of artificially Wolbachia-infected mosquitoes and the mechanisms of interference between Wolbachia, pathogens and hosts.
Acknowledgements
We would like to present gratitude to King Abdulaziz City for Science and Technology, Ryidh, Saudi arabia for their technical support.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
There is no funding source.
References
- Chen LH, Wilson ME (2010) Dengue and chikungunya infections in travelers. Curr Opin Infect Dis 23: 438–444
- World Health Organization (WHO) (2019). Dengue and severe dengue. 2019 https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- Lima, E.P., Paiva, M.H.S., Paula de Araújo, A., da Saliva, U. M. da Saliva, de Oliveira, L. N., et al. (2011)Insecticide resistant in Aedes aegypti populations from ceara, Brazil. Parasites & Vectors, 4(1), 5.
- Marcombe, S., Mathieu, R.B., Pocquet, N., Riaz, M.-A, Poupardin, R., Selior, S., et al. (2012) Insecticide resistant in the dengue vector Aedes aegypti from Matrinique: Distribution, mechanisms and relations with environmental factors, PLoS One, 7 (2), Article e30989
- Achee, N. L., Gould, F., Perkin, T.A., Reiner Jr. R.C., Morrison, A.C., Ritchie, S.A. et al. (2015) critical assessment of vector control for dengue prevention, PLoS Neglected Tropical Diseases, 9 (5) e0003655
- WHO (1998) Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, BioEfficacy and Persistence of Insecticides on Treated Surfaces. Report of the WHO informal consultation on WHO/HQ (document WHO/ cDS/cpc/MaL/98.12), 28–30 September. geneva, Swizerland: World Health Organization
- Zaim M, Guillet P. (2002) Alternative insecticides: an urgent need. Trends in Parasitology, 8(4):161–163.
- Kay B. and Nam VS. (2005) New strategy against Aedes aegypti in Vietnam. The Lancet. 365(9459):613–617.
- Yakob L. and Walker T. (2016) Zika virus outbreak in Americas: The need for novel mosquito control methods, The Lancet Global Health, 4 (3), pp. e148-e149
- Labbé G.M.C., Scaife S., Morgan, S.A., Curtis Z. H., Alphey L. (2012) Female-specific flightless (fsRIDL) phenotype for control of Aedes albopictus, PLoS Neglected Tropical Diseases, 6(7), e1724
- Ferguson N.M., Kien D. T. H., Clapham H., Augas R., Vu T.T., Chau, T.N.B., et al. (2015) Modeling the impact on virus transmission of wolbachia-mediated blocking of dengue virus infection of Aedes aegypti, Science Translational Medicine, 7 (279)
- Kamtchum-Tatuene, J. et al. (2017) “The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections,” Current opinion in infectious diseases. Wolters Kluwer Health, 30(1), p. 108.
- Hoffmann, A. A., Montgomery B. L., Popovici, J., Iturbe-Ormaetxe I., Johnson P.H., Muzzi F., et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission, Nature, 476 (7361), p.454
- Frentiu F.D., Zakir T., Walker T., Popovici J., Alyssa T.P., van den Hurk A., et al. (2014) Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia, PLoS Neglected Tropical Diseases, 8(2) p. e2688
- O’Neill, S.L., Ryan P.A., Turley, A. P., Wilson G., Retzki K., Iturbe-Ormaetxe I., et al. (2018) Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses, Gates Open Research, 2
- Van den Hurk, A.F., Hall-Mendelin, S., Pyke, A.T., Frentiu, F.D., McElroy, K., Day, A., Higgs, S. and O’Neill, S.L. (2013) Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Pathogens 6, e1892.
- Kambris, Z., Blagborough, A.M., Pinto, S.B., Blagrove, M.S., Godfray, H.C., Sinden, R.E. and Sinkins, S.P. (2010) Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles PLoS Pathogens 6, e1001143.
- Hughes, G.L., Koga, R., Xue, P., Fukatsu, T. and Rasgon, J.L. (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathogens 7, e1002043.
- Aliota M. T., Peinado S. A., Velez, I. D. and Osorio J. E. (2016) The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti, Scientific Reports, 6, p. 2879
- Popovici, J., Moreira, L.A., Poinsignon, A., Iturbe-Ormaexte, I., McNaughton, D. and O’Neill, S.L. (2010) Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes Memorias do Instituto Oswaldo Cruz 105, 957–964.
- Murphy, B., Jansen, C.C., Murray, J. and De Barro, P. (2010) Risk Analysis on the Australian Release of Aedes aegypti (L.) (Diptera: Culicidae) Containing Wolbachia. CSIRO, Brisbane, Australia.
- Heath BD, Butcher DJ, Whitfield WgF, Hubbard SF (1999) Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biology 9: 313–316
- Huigens ME, de almeida rp, Boons pa, Luck rF, Stouthamer r (2004) Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc Royal Soc Lond B Biol Sci 271: 509–515
- Klasson L, Kambris z, cook pE, Walker t, Sinkins Sp (2009a) Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics 10: 33
- McNulty SN et al (2010) Endosymbiont DNa in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer. PLoS ONE 5: e11029
- Rances, E., Ye, Y.H., Woolfit, M., McGraw, E.A. & O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLOS Pathogens 8(2): e1002548.
- Laven, H. (1967) Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216, 383–384.
- Scott, R. (2014). Rear and release: a new paradigm for dengue control. Austral Entomology 53: 363-367.
- Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti Nature, 476(7361):450–453.
- Nazni, W. A., Hoffmann, A. A., NoorAfizah et al. (2019) Establishment of Wolbachia strain wAlbB in Malaysian Populations of Aedes Aegypti for Dengue control, Current Biology 29, 4241–4248
- Schmidt TL, Barton NH, Rašić G, et al. (2017) Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 15(5): e2001894.
- Adekunle, A. I., Meehan, M. T., and McBryde, E. S. (2019) Mathematical analysis of a Wolbachia invasive model with imperfect maternal transmission and loss of Wolbachia infection, Infectious Disease Modelling xxx (xxxx) xxx-xxx (https://doi.org/10.1016/j.idm.2019.10.001)
- M. (2015) An introduction to mathematical epidemiology, volume 61. Springer, 2015.
- Rafikov, M., Meza, M. E., Correa, D. P. and Wyse. A. P. (2019) Controlling Aedes aegypti populations by limited wolbachia -based strategies in a seasonal environment. Mathematical Methods in the Applied Sciences, mar 2019. doi: 10. 1002/mma.5527. URL https://doi.org/10.1002/mma.5527.
- Campo-Duarte, D. E., Vasilieva, O., Cardona-Salgado, D. and Svinin M. (2018) Optimal control approach for establishing wmelpop wolbachia infection among wild aedes aegypti populations. Journal of mathematical biology, 76(7):1907–1950, 2018.
- Zheng, B., Tang, M., Yu, J. and Qiu. J. (2018) Wolbachia spreading dynamics in mosquitoes with imperfect maternal transmission. Journal of mathematical biology, 76(1-2): 235–263.
- Caspari E. and Watson G. (1959) On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution, 13(4):568–570.
- Ndii M. Z., Hickson, R. I. and Mercer G. N. (2012) Modelling the introduction of wolbachia into Aedes aegypti mosquitoes to reduce dengue transmission. The ANZIAM Journal, 53(3):213–227.
- Xue L., Manore, C. A., Thongsripong, P. and Hyman M. (2017) Two-sex mosquito model for the persistence of wolbachia. Journal of biological dynamics, 11(sup1):216–237.
- Qu, Z., Xue, L. and Hyman J. M. (2018). Modeling the transmission of wolbachia in mosquitoes for controlling mosquito-borne diseases. SIAM Journal on Applied Mathematics, 78(2):826–852.
- Li, Y. and Liu X. (2017) An impulsive model for wolbachia infection control of mosquito borne diseases with general birth and death rate functions. Nonlinear Analysis: Real World Applications, 37:412–432.
- ƠReilly, K. M., Hendrickx, E., Kharisma, D. D. et al. (2019) Estimating the burden of dengue and the impact of release of wMel Wolbachia –infected mosquitoes in Indonesia: a modelling study, BMC Medicine 17:172
- Jeffries, C. L., Lawrence, G. G., Golovko, G. et al. (2018) Novel Wolbachia strains in Anopheles malaria vectors from Sub-Saharan Africa, Wellcome Open Research 3:113
- Atyame, C. M. et al. (2016) “Comparison of irradiation and Wolbachia based approaches for sterile-male strategies targeting Aedes albopictus,” PLoS one. Public Library of Science, 11(1).
- Blagrove, M. S. C. et al. (2012) “Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus,” Proceedings of the National Academy of Sciences. National Acad Sciences, 109(1), pp. 255–260.
- Alphey, L. et al. (2013) “Genetic control of Aedes mosquitoes,” Pathogens and global health. Taylor & Francis, 107(4), pp. 170–179.
- Atyame, C. M. et al. (2015) “Wolbachia-based population control strategy targeting Culex quinquefasciatus mosquitoes proves efficient under semi-field conditions,” PloS one. Public Library of Science, 10(3).
- Lacroix, R. et al. (2012) “Open field release of genetically engineered sterile male Aedes aegypti in Malaysia,” PloS one. Public Library of Science, 7(8), p. e42771.
- Niang, E. H. A. et al. (2018) “Biological Control of Mosquito-Borne Diseases: The Potential of Wolbachia-Based Interventions in an IVM Framework,” Journal of tropical medicine. Hindawi.
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell. 139(7): 1268–1278.
- Moreira LA, Saig E, Turley AP, Ribeiro JMC, O’Neill SL, McGraw EA. (2009) Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Neglected Tropical Diseases, 3(12):e568.
- Sinkins SP, O’Neill SL. (2000) Wolbachia as a vehicle to modify insect populations. In: Handler A, James AA, eds. Insect transgenesis: methods and applications. Boca Raton: CRC Press, pp. 271–287.
- Aksoy S, Cook P, McMeniman C, O’Neill S. (2008) Modifying insect population age structure to control vectorborne disease, in transgenesis and the management of vector-borne disease. New York: Springer, pp.126–140.
- Bian G, Xu Y, Lu P, Xie Y, Xi Z. (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathogens, 1;6(4):e1000833.
- Glaser, R. L. and Meola, M. A. (2010) “The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection,” PloS one. Public Library of Science, 5(8).
- Dodson, B. L. et al. (2014) “Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis,” PLoS neglected tropical diseases. Public Library of Science, 8(7).
- Dutra, H. L. C. et al. (2016) “Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes,” Cell host & microbe. Elsevier, 19(6), pp. 771–774.
- Fakeeh M. and Zaki A. M. (2001) Virologic and Serologic Surveillance for Dengue Fever In Jeddah, Saudi Arabia, 1994–1999, Am. J. Trop. Med. Hyg., 65(6), 764–767.

This work is licensed under a Creative Commons Attribution 4.0 International License.





