Manuscript accepted on : 12 December 2017
Published online on: --
Swathi P1, Gana Manjusha K2, Vivekanand M3, Ramkishan A4 and Bhavani B4
1,3A.U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, India.
2Vignan Institute of Pharmaceutical Technology, Visakhapatnam, India.
4Deputy drug controller of India, CDSCO.
Corresponding Author E-mail: bhavani2008@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2589
ABSTRACT: The present study was designed to investigate the antihyperglycemic and hypolipidemic activities of ethanolic leaf extract of Morus alba (EMA) in Streptozotocin induced diabetic rats. Blood glucose, serum parameters such as glycated haemoglobin (HbA1c), bilirubin, albumin, creatinine, total protein, urea, lipid profile, and urine parameters such as urine protein, creatinine and volume and renal antioxidant enzymes like SOD, CAT, GSH and MDA were estimated at the end of 12 weeks study period. Kidney histopathology was also done. The treatment with EMA showed significant (p<0.05) reduction in the elevated blood glucose, HbA1c, kidney function parameters and lipid profile in STZ induced diabetic rats. Treatment with EMA exerted improvement in antioxidant enzymes as SOD, CAT, GSH and reduction in MDA level profile in STZ induced diabetic rats. Histopathology reveals, EMA showed marked amelioration of glomerulosclerosis caused by STZ. The activities of Morus alba might be due to the presence of antioxidant principles like terpenoids and sterols.
KEYWORDS: Diabetes; Blood glucose; Hyperglycemia; Hyperlipidemia Nephropathy;
Download this article as:| Copy the following to cite this article: Swathi P, Manjusha G. K, Vivekanand M, Ramkishan A, Bhavani B. Effect of Morus Alba Against Hyperglycemic and Hyperlipidemic Activities in Streptozotocin Induced Diabetic Nephropathy. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Swathi P, Manjusha G. K, Vivekanand M, Ramkishan A, Bhavani B. Effect of Morus Alba Against Hyperglycemic and Hyperlipidemic Activities in Streptozotocin Induced Diabetic Nephropathy. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28473 |
Introduction
Diabetes mellitus is a chronic and progressive metabolic disease characterized by hyperglycemia due to insulin deficiency, or resistance, or both. Besides hyperglycemia, several other symptoms, including hyperlipidemia, are involved in the development of microvascular and macrovascular complication of diabetes. According to the latest survey in 219 countries, among people egad 20 to 79 years, 382 million people have diabetes mellitus and it is estimated that this number will reach 592 million in 2035.1 Diabetic nephropathy (DN) is a major cause of end stage renal failure worldwide. DN has not been traditionally considered an inflammatory disease; however, recent studies have shown that kidney inflammation is crucial in promoting the development and progression of DN.2
Reactive oxygen species play an important role in high glucose induced renal injury.3 Free radicals are capable of damaging cellular molecules, DNA, proteins, and lipids leading to altered cellular functions. Many recent studies reveal that antioxidants capable of neutralizing free radicals are effective in preventing experimentally induced diabetes in animal models.4
Herbal medicines are naturally occurring; plant-derived substances with minimal or no industrial processing that have been used to treat illness within local or regional healing practices. For a long time, herbal medicines or their extracts have been used to cure various diseases, because plant products are frequently considered to be less toxic and free from side effects than synthetic ones. Therefore, studies with plant extracts are useful to understand their efficacy, mechanism of action and safety for treatment and management of diabetes.
Numerous plants have been reported to possess hypoglycemic activity and diabetic nephropathy. Some reported plant sources are Ginkgo biloba, Fragaria ananassa, Moringa oleifera, Cladophora glomerata, Garcinia indica, Ocimum Gratissimum, Astragalus membranaceus and Trigonella foenumgracum.5-9 Reports indicated herbal inventions play a pivotal role in the treatment of diabetes and diabetic nephropathy.
The present investigation was focused on Morus alba (Moraceae). Morus alba reported pharmacological activities related to in vitro antioxidant activity,10 hypoglycemic activity in alloxane induced diabetic rats as α-amylase inhibitory activity11 and protective action on ocular function12 due to the presence of triterpenes (lupeol) Sterols (β- Sitosterol), bioflavonoids (rutin, moracetin, quercetin-3-triglucoside and isoquercitrin), coumarins, volatile oil, alkaloids, amino acids and organic acids. Morus alba leaves contain rutin, quercetin and apigenin as bioactive constituents.13 Considering the antioxidant activity and hypoglycemic properties of Morus alba, this study was designed to evaluate the ameliorator effect of Morus alba on oxidative stress induced diabetic nephropathy in diabetes rats induced by Streptozotocin.
Materials and Methods
Plant Material
Fresh leaves of Morus alba (Mulburry) were obtained from the rural areas of Visakhapatnam and identified and authentified by plant taxonomist, department of Botany, Andhra University, India.
Preparation of Plant Extract
The fresh leaves are washed and cut into small pieces and dried under sunshade for 6−7 days and coarsely powdered. The powder was extracted using soxhelt apparatus with ethanol 2000 ml. The methanol was distilled condensed using rotatory evaporator and stored in desicator. The powder of the extract was suspended in appropriate solvent system
Chemicals
Streptozotocin was procured from Sigma Co. USA, metformin from USV Limited, Maharashtra, while other chemicals used were of analytical grade obtained from E. Merck and Hi-media, India.
Animals and Treatment
Male Wistar rats (150–180 gm) were used. These were bred in our animal facility and housed in an air-conditioned room (approximately 22 °C) with controlled lighting 12:12 h light/dark cycle. The animals were maintained with pelleted, while tap water was available ad libitum. The study has got the clearance from the Institutional Animal Ethical Committee (IAEC) the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Rats were acclimatized to the environment for 15 days prior to the experiment; animals were divided into five groups. Each group contains 6 rats. Fasted animals were deprived of food for at least 16 hr but allowed free access to water. The first group was used as control and received H2O as vehicle. The second group received a single dose of STZ (60 mg/kg b.wt) and dissolved in citrate buffer and was divided into four subgroups after establishing of the diabetes for 1 week. The first subgroup was kept as a diabetic control while the second, third, and fourth subgroups received orally 1.0 ml of metformin (500mg/kg b.wt), EMA (150 mg/ kg) and EMA (300 mg /kg) respectively by gastric intubation daily for 12 weeks.
Preparation of Samples
The rats were then etheranesthetized, decapitated and blood sample collected in plain and heparined tubes. From the clotted blood at room temperature, serum was collected after centrifugation (3000 rpm, 10 min) and used to determine the biochemical parameters. Twenty four hour urine samples were collected using metabolic cages and analyzed. The animals were kept individually in metabolic cages, and they were given only water.
The kidney were removed, weighed and placed immediately in ice-cold buffer (0.25M sucrose, 10mM Tris and 0.3 mM EDTA; pH 7.4) and washed thoroughly with distilled water to remove blood. The kidneys were homogenized using a Teflon homogenizer. The total homogenate was used for estimation of lipid profile and MDA. The homogenate was centrifuged (15 min, 15 000 rpm), the whole supernatant removed and frozen at -20 ºC for estimation of antioxidant enzymes.
Biochemical Estimations
Fasting blood glucose levels were estimated by using GOD/POD method.14 Glycosylated haemoglobin15 cholesterol and triglyceride (TG) levels and HDL-cholesterol by kit method, bilurubin,16 total protein17 and albumin18 were also estimated. At the end of the 12 week treatment period rats were sacrificied and kidneys were used for histopthological examination.
Enzyme Estimations in Kidney
Superoxide dismutase was measured according to the method described by Kakkar et al., 198419; catalase was estimated by the method of Sinha, 1972,20 reduced glutathione measured by method of Jollow et al., 197421 and melanoldehyde was measured by the method of Ohkawa et al., 1979.22
Histopathology
Renal tissues were collected after animal sacrifice, fixed in 10% formalin, processed routinely, and embedded in paraffin. 5 𝜇m thick sections were prepared and stained with hematoxylin and eosin (H&E) dye for microscopic investigation.23 The stained sections were examined and photographed under a light microscope.
Statistical Analysis
Values are mean±SEM for six rats in each group, and significance of the differences between mean values was determined by Bonferroni-post test. The levels of significance were evaluated with p values.
Results and Discussion
Tight control of blood glucose can reduce clinical complications in diabetic patients. However, alternative treatment strategies are required to prevent the oxidative stress complications such as diabetes. It is well documented that modulations of oxidative stress through treatment with antioxidants can effectively reduce the development of diabetes.24
In the present study, diabetes mellitus was induced in rats through a STZ injection that causes the destruction of β-cells of islets of Langerhans, as proposed by many authors.24 This effect was represented in the current study through the elevation of blood glucose in STZ induced diabetic control rats. The administration of EMA showed significant (p<0.05) reduction of blood glucose levels (Table 1) accompanied with reduction in glycosylated heamoglobin levels (Table 2).
Table 1: Effect of Morus alba on blood glucose levels in STZ induced diabetic rats during different intervals of 12 weeks study period.
| Treatment (mg/kg) | 0day | 3 week | 6 week | 9 week | 12 week |
| Control | 108.23±2.71 | 97.16±1.21 ns | 101.66±3.19 ns | 99.33±2.03 ns | 96.23±4.79 ns |
| D.Control | 325±4.93 | 316.45±4.50 ns | 333.16±5.66 ns | 319.16±5.23 ns | 324.83±4.11ns |
| Standard | 321.11±6.53 | 213.13±2.18* | 134.23±3.17** | 109.32±2.36 *** | 92.50±2.99*** |
| EMA (150mg/kg) | 297.65±3.68 | 223.83±4.23* | 146.35±3.60** | 121.33±3.99*** | 98.60±1.66*** |
| EMA (300mg/kg) | 314.13±4.88 | 198.66±3.14** | 126.46±1.84** | 98.13±4.16*** | 87.26±4.55*** |
Number of rats per group = 6; data on each parameter were analyzed by one-way ANOVA followed by Dunnet’s test;
*P< 0.05, **P< 0.01, *** P< 0.001 as compared with diabetic control
Hypercholesterolemia, hypertriglyceridemia and enhanced glomerular lipid synthesis have been implicated in diabetic glomerulosclerosis and known to exercebate kidney diseases. Several groups of hypoglycaemic drugs are currently available to treat Diabetes. The abnormal high concentration of serum lipids in diabetes is mainly due to the increase in the mobilization of free fatty acids from the peripheral depots since insulin inhibits the hormone sensitive lipase.4 In EMA treated diabetic rats shown to normalize the lipid levels (reduction of TG, TC, LDL, VLDL and elevation of HDL) as compared to the STZ induced hyperlipidemia (elevation of TG, TC, LDL, VLDL and reduction of HDL both in serum and kidney homogenate as shown in Figure 1 and Figure 2. The hyperglycemic effect of EMA could indirectly be related to beneficial action against diabetic hypercholesterolemia, hypertriglyceridemia.
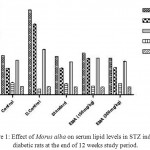 |
Figure 1: Effect of Morus alba on serum lipid levels in STZ induced diabetic rats at the end of 12 weeks study period. Click here to View figure |
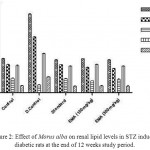 |
Figure 2: Effect of Morus alba on renal lipid levels in STZ induced diabetic rats at the end of 12 weeks study period. Click here to View figure |
Diabetic nephropathy in uncontrolled diabetes is a serious micro-vascular complication leading to glycosylation of renal basement membranes and result in hyperproteinuria, hypercreatinemia, and increased urinary excretion of creatinine, urea, and uric acid.25 Normally, the kidney excretes creatinine and only low amount of low-molecular weight protein passes through the glomerulus,26 whereas urea and uric acid are reabsorbed by the proximal tubule. EMA showed significant (p<0.05) reduction of bilirubin, creatinine and elevation of protein levels than those of nondiabetic group, implying the presence of diabetic kidney disease with renal hyperfiltration due to STZ induction (Table 2). We observed that repeated treatment with EMA could attenuate hyperproteinuria, hypercreatinemia as well as ameliorate the loss of renal function and glomerular hyperfiltration in STZ-diabetic rats. Due to improved the filtration function and tubular reabsorption by EMA, urinary creatinine, urea, and uric acid level were controlled significantly p<0.05 (table 3).
Table 2: Effect of Morus alba on serum parameters in STZ induced diabetic rats at the end of 12 weeks study period.
| Treatment (mg/kg) | HbA1c (%) | T.bilirubi (mg/dL) | Creatinine (mg/dL) | Total protein (mg/dL) | Albumin (mg/dL) |
| Control | 4.09±0.23** | 0.72±0.016* | 0.68±0.02* | 6.93±0.14* | 3.35±0.09* |
| D.Control | 11.43±0.56 | 1.78±0.042 | 1.92±0.03 | 3.88±0.34 | 7.20±0.10 |
| Standard | 3.42±0.16*** | 0.77±0.025* | 0.69±0.02 * | 6.13±0.67* | 3.86±0.06* |
| EMA (150mg/kg) | 5.02±0.25* | 0.92±0.016 * | 0.89±0.04 * | 7.90±0.24* | 5.13±0.11* |
| EMA (300mg/kg) | 4.16±0.54** | 0.73±0.013 * | 0.71±0.03** | 6.06±0.18* | 3.99±0.12** |
Number of rats per group = 6; data on each parameter were analyzed by one-way ANOVA followed by Dunnet’s test;
*P< 0.05, **P< 0.01, *** P< 0.001 as compared with diabetic control
Table 3: Effect of Morus alba on urine parameters in STZ induced diabetic rats at the end of 12 weeks study period
| Treatment (mg/kg) | Creatinine (mg/dL) | Urine volume (ml/24hrs) | Ccr (ml/hrs) | Uric acid (mg/dL) | Urea (mg/dL) |
| Control | 0.61±0.05 | 09.42±0.41 | 1.31±0.79 | 4.09±0.32 | 22.03±0.77 |
| D.Control | 1.68±0.03 | 26.52±0.85 | 6.61±0.49 | 8.72±0.18 | 39.46±0.94 |
| Standard | 0.69±0.04** | 14.43±0.43* | 1.84±0.11** | 4.23±0.34* | 26.89±0.77** |
| EMA (150mg/kg) | 1.11±0.04 * | 18.17±0.32* | 2.73±0.20* | 5.79±0.21* | 32.23±0.93* |
| EMA (300mg/kg) | 0.65±0.04 ** | 12.09±0.49* | 1.89±0.21** | 4.96±0.44* | 27.13±0.87** |
Number of rats per group = 6; data on each parameter were analyzed by one-way ANOVA followed by Dunnet’s test;
*P< 0.05, **P< 0.01, *** P< 0.001 as compared with diabetic control
Oxidant stress markers (CAT, SOD, GSH and MDA), which are critical factors in the progression of diabetic nephropathy, are increased by inflammatory mechanisms of injury in the kidney.27 Among antioxidative enzymes, SOD catalyzes dismutation of the superoxide anion into hydrogen peroxide, while GSH both detoxifies hydrogen peroxides and converts lipid hydroperoxides to nontoxic alcohols; thus, antioxidant enzymes activities could reflect antioxidant defense status.28 In this work, the reduced activities of SOD, CAT and GSH in kidney of STZ-diabetic rats were elevated significantly (p<0.05) by EMA (table 4). The classical formula protecting kidney of diabetic rats from oxidative damage by enhancing enzymatic antioxidative defense systems could be considerable. A significant increase in MDA, an index of endogenous lipid peroxidation, has been shown under diabetic conditions.29 From this view point, the prevention of oxidative stress-related hyperlipidemia and/or lipid peroxidation is considered crucial in preventing disorders associated with diabetes.30 s The EMA showed significant reduction in MDA levels in kidney homogenate of STZ induced diabetic rats as shown in table 4.
Table 4: Effect of Morus alba on antioxidant parameters in STZ induced diabetic rats at the end of 12 weeks study period
| Treatment (mg/kg) | SOD (U/mg of protein) | CAT (U/mg of protein) | GSH (mg/100g tissue) | MDA (U/mg of protein) |
| Control | 10.23±1.23 | 34.23±1.86 | 20.46±0.37 | 132.16±0.88 |
| D.Control | 4.65 ±1.01 | 17.64±1.34 | 15.65±0.17 | 246.79±2.13 |
| Standard | 9.46±0.89** | 32.16±0.68*** | 21.13±0.26*** | 139.23±3.77*** |
| EMA (150mg/kg) | 7.26±0.74* | 28.42±0.97** | 20.11±0.41** | 221.85±2.47* |
| EMA( 300mg/kg) | 10.16±1.06*** | 31.09±1.35** | 20.16±0.74*** | 143.92±0.49*** |
Number of rats per group = 6; data on each parameter were analyzed by one-way ANOVA followed by Dunnet’s test; *P< 0.05, **P< 0.01, *** P< 0.001 as compared with diabetic control
The light microscopic examination of kidney of disease control group revealed the altered structure bowman’s capsule by increasing bowman’s space and presence of cellular infiltrates. The treatment with the EMA extract and metformin found to prevent the degenerative changes in STZ induced diabetic rats (figure 3). It is reported several phytochemicals triterpenes, β- Sitosterol, bioflavonoids coumarins and volatile oils might be responsible for the antioxidant, antihyperglycemic and hyperlipidemic activities of Morus alba.
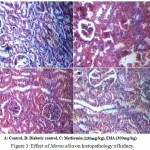 |
Figure 3: Effect of Morus alba on histopathology of kidney. Click here to View figure |
3A: Normal glomeruli and a compact tissue appearance with bowmans capsule (BC)3B: increased bowmans space (BS) and presence cellular infiltrates (CI) indicates celluar damage. 3C: Noticeable improvements in the tissue architecture were evident, and more visible glomeruli and fewer inflammatory cells were observed.4D: absence of inflammatory cells, normal basement membrane.
Conclusion
In conclusion, Morus alba decreased blood glucose levels (hypoglycemia), normalizing the lipid levels in both serum and kidney (dyslipidemia), reduction in both serum and urine parameters and increased antioxidant enzymes activities (antioxidant) in diabetic rats. And consequently could alleviate diabetic nephropathy.
Conflict of Interest
Authors declare no conflicts of interest.
References
- Guariguata L, Whiting D.R, Hambleton I. Global estimates of diabetes prevalence for 2013 and projections for 2035. Res. Clin. Prac. 2014; 103(2):137–149.
CrossRef - Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. 2013;124:139–152.
- Bonnefont D, Bastard J.P, Jaudon M.C, Delattre J. Consequences of diabetes status on the oxidant /antioxidant balance. Metabol. 2000;26:163−76.
- Swathi P, Kumar J.T, Kumar E.K. Methanolic fruit extract of Garcinia indica ameliorates progression of diabetic nephropathy in Streptozotocin induced diabetic rats. Int J Bio Pharmaceu Res. 2015;6(1):12−18.
- Lu Q, Zuo W.Z, Ji X.J. Ethanolic Ginkgo biloba leaf extract prevents renal fibrosis through Akt/mTOR signaling in diabetic nephropathy. Phytomedicine. 2015;22(12):1071-8.
CrossRef - Ibrahim D.S, Abd M.A. Effect of strawberry (Fragaria × ananassa) leaf extract on diabetic nephropathy in rats. Int J Exp Pathol. 2015;96(2):l87−93.
- Abdulrahman L, Al M, Haddad A.E.R. The Antidiabetic Effect of Low Doses of Moringa oleifera Lam. Seeds on Streptozotocin Induced Diabetes and Diabetic Nephropathy in Male Rats. Bio Med Res Int. 2015;Article ID 381040:13.
- Yingli J, Yan S, Yinggang Z, Chunsheng M. Fenugreek Prevents the Development of STZ-Induced Diabetic Nephropathy in a Rat Model of Diabetes. Evi Based Comple Alter Med. 2014; Article ID 259368:11.
- Chutima S, Atcharaporn O, Naruwan S, Pornpun V,Anchalee P, Doungporn A, Sunhapas S, Varanuj C. Antidiabetic and renoprotective effects of cladophora glomerata kützing extract in experimental type 2 diabetic rats: A potential nutraceutical product for diabetic nephropathy. J Diabetes Res. 2015; Article ID 320167:15.
- Chon S.U, Kim Y.M, Park Y.J, Buk-Gu H, Yong-Seo P, Shela G. Antioxidant and antiproliferative effects of methanol extracts from raw and fermented parts of mulberry plant (Morus alba L.). Eur Food Res Technol. 2009;230:231−237.
CrossRef - Bahman N, Golboo M. Influence of Three Morus Species Extracts on α-Amylase Activity. Iran J Phar Ceu Res. 2009;8(2):115−119.
- El-Sayyad H.I, Sherbiny H. A, Sobh M.A, Abou-El-Naga A.M, Ibrahim MA, Mousa S.A. Protective effects of morus albaleaves extract on ocular functions of pups from diabetic and hypercholesterolemic mother rats. Int J Biol Sci. 2011:7(6):715-728.
CrossRef - Doi K, Kojima T, Makino M, Kimura Y, Fujimoto Y. Studies on the constituents of the leaves of Morus alba L. Chem Pharm Bull. 2001;49:151-53
CrossRef - Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22(2):158-61.
CrossRef - Sudhakar N.S, Pattabiraman T.N. A new colorimetric method for the estimation of glycosylated haemoglobin. Clinica. Chimica Acta. 1981; 109:267–274.
CrossRef - Jendrassik L, Grof P. Colorimetric Method of Determination of bilirubin. Biochem Z. 1938;297:81-82.
- Tietz N.W. Fundamentals of Clinical Chemistry, Philadelphia, W.B. Saunders Company. 1996;240.
- Doumas B.T, Arends R.I, Pinto P.C. Standard methods of Clinical Chemistry. Academic Press Chicago. 1972;7:175-189.
- Kakkar P, Ballabh D, Vshwanathan P.N. A modified spectrophotometric assay of superoxidesidumate. J. Biochem. Biophy. 1984;21:130−132.
- Sinha A.K. Colourimetric assay of catalase. Analyt Biochem. 1972;47: 389−394.
CrossRef - Jollow D.J, Michell J.R, GilleteJ.R. Bromoibenzene-induced Liver necrosis: Protective role of glutathione and evidence for 3,4- Bromobenzene oxide as hepatotoxic metabolite. Pharmacology. 1974; 11:151-169.
CrossRef - Drury R.A, Wallington E.A, Cancerson R. Carlton’s Histopathological Techniques, Oxford University Press, Oxford, UK, 4th edition. 1976;345-789.
- Ahmad N.K, Rahmat A.K, Mushtaq A, Nadia M. Role of antioxidant in oxidative stress and diabetes mellitus. J Phar Cog Phytochem. 2015; 3(6):217-220.
- Proctor G, Jiang T, Iwahashi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and Ove 26 mice with type 1 diabetes. Diabetes. 2006;55:2052-2509.
CrossRef - Frey J, Daudon M, Raby N. Valeur séméiologique des paramètres biochimiques urinaires. Ann Biol Clin. 2001;59:13-25.
- Almdal T.P, Vilstrup H. Strict insulin treatment normalizes the organic nitrogen contents and the capacity of urea–N synthesis in experimental diabetes in rats. Diabetologica. 1988;31:114–8.
CrossRef - Ohtake T, Kimura M. Roles of reactive oxygen species and antioxidant enzymes in murine daunomycin-Induced nephropathy. J Lab Clin Med. 1997;129:81−88.
CrossRef - Seifried H.E, Anderson D.E, Fisher E.I. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nut Biochem. 2007;18(9):567–579.
CrossRef - Janero D.R, Burghardt B. Analysis of cardiac membrane phospholipid peroxidation kinetics as malondialdehyde: nonspecificity of thiobarbituric acid-reactivity. Lipids. 1988;23:452–8.
CrossRef - Schrijvers B.F, Vriese A.S. Novel insights in the treatment of diabetic nephropathy. Acta Clin Belg. 2007;62:278–90.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





