Manuscript accepted on : 21 February 2017
Published online on: --
Plagiarism Check: Yes
Ayat Badr Al-Ghafari and Ghrob Hussein Althaqafi
Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia.
Corresponding Author E-mail: abalghafari@kau.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2418
ABSTRACT: Coffee is considered as one of the most consumed beverages worldwide and a major source for antioxidants. This study examines the antioxidant activity of water extracts from two coffee types (ground and instant), which were prepared according to the basis of their serving size. Using several colorimetric assays, the hydrogen peroxide and DPPH scavenging, as well as metal ion chelating activity and reducing power for the two coffee types were assessed. Results showed that ground coffee had more total phenolic content than instant coffee. However, extract from instant coffee showed a higher antioxidant activity especially as a scavenging and reducing agent than ground coffee. On the other hand, metal chelating activity of ground coffee extract was higher than the instant coffee extract. The differences in antioxidant activities between the two coffee brews extracts might be due to processing conditions especially roasting degrees and geographical sources.
KEYWORDS:
DPPH antioxidant activity; ground coffee; instant coffee; metal chelating reducing power; scavenging activity;
Download this article as:| Copy the following to cite this article: Al-Ghafari A. B, Althaqafi G. H. The effect of Processing and Roasting on the Antioxidant Activity of extracts from instant and ground Coffee. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Al-Ghafari A. B, Althaqafi G. H. The effect of Processing and Roasting on the Antioxidant Activity of extracts from instant and ground Coffee. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21982 |
Introduction
Worldwide, coffee is one of the most preferred and commonly consumed beverages. Coffee beans that are present in markets are produced from two different species of coffee either Arabica or Robusta or from mixing both beans types in different percentages.1 Coffee has a wide range of important polyphenol antioxidants, phenolic acids, and flavonoids, which are important to human health from several prospective.1 From this point of view, researches are focused on the possible beneficial effects of coffee.2,3 Most researchers suggest that the strong antioxidant activity present in roasted coffee are due to the presence of compounds known as polyphenols such as chlorogenic acids, which are naturally present in green coffee beans or formed as a product from Maillard process. It is well studied that the geographical origins of coffee beans as well as the roasting processes, may affect the antioxidant activity and may change the seed composition which in turn might destroy some important compounds in coffee.1,4,5 Therefore, the major objectives of this study were to investigate antioxidant activities of the water extracts of ground and instant coffees that are most commonly consumed in Jeddah, western region of Saudi Arabia. In the current study, the preparation of water extracts was performed based on the serving size of the two coffee types. Then, the total polyphenol and flavonoid contents and the antioxidant activities were examined for the two types of coffee using different antioxidant colorimetric assays.
Materials and Methods
Preparation of Coffee Samples
In this study, two major types of coffee, ground and instant, were chosen based on an electronic survey performed on 900 participants to determine the preferred coffee types and brands that are mostly consumed in Jeddah, Saudi Arabia. The most consumed ground and instant coffees from the survey were Turkish coffee and Nescafé red mug, respectively. Both samples were obtained from local coffee market in Jeddah, Saudi Arabia and were prepared according to their serving size. The tested concentration of the ground coffee (Turkish coffee) was 3.5g in 75ml warm water,6 whereas, the tested concentration of the instant coffee (Nescafé red mug) was 2g in 200ml boiled water.3
Determination of total Polyphenol and flavonoid contents
The traditional Folin-Ciocalteau method was applied to assess the total polyphenol content (TPC) of the coffee samples with some modification.7 In a dark place, coffee extract was incubated for 1 hour with the Folin-Ciocalteu reagent (BDH, Poole, UK) and sodium carbonate (2%) (CDH, New Delhi, India). Then, the absorbance was measured at 750 nm and a calibration curve was prepared to determine the polyphenol contents from different concentrations of Gallic acid (GA) (Sigma-Aldrich, Poole, UK) (100, 200, 300, 400, 500, 600, 700 and 800 µg/ml) diluted in methanol: water (1:1v/v) as a standard solution.
Regarding the determination of total flavonoid content (TFC), aluminum chloride assay was performed. 8 Coffee extracts were mixed with sodium nitrate (Sigma-Aldrich, Poole, UK), deionized water, and were incubated at room temperature for 5 minutes. Then, a mixture of 10% aluminum chloride (LOBA, Mumbai, India), (1M) Sodium hydroxide (PanReacAppliChem, Barcelona, Spain) and deionized water was prepared and was added to the coffee extract samples. The final absorbance of the mixture was measured at 510 nm and a calibration curve was made from various concentrations of (+)-Catechin (CA) (Steinheim, Germany) (50,100,150,200,250,300 and 500 µg/ml).
Determination of radical-scavenging activity with DPPH assay
The free radical scavenging activity of the coffee extracts was examined by assessing the stability of 2,2 diphenyl-1-picrylhydrazyl (DPPH).9 The coffee sample extract was added to ethanol (99.5%) (Sigma-Aldrich, Poole, UK), DPPH reagent (Sigma-Aldrich, Poole, UK) and was left for 1 hour in dark place before the final absorbance was recorded at 517 nm. The percentage inhibition of DPPH free radical scavenging activity was subsequently calculated using a simple equation:
Percentage inhibition= [(A0-A1)/A0]*100, Where A0 is the absorbance of blank sample, and A1 is the absorbance of the coffee sample.
Determination of Hydrogen Peroxide (H2O2) Scavenging activity
To determine the ability of coffee extracts to scavenge hydrogen peroxide,10 the water extract of the coffee sample was added to 40 mM hydrogen peroxide diluted in phosphate buffer saline at pH 7.4 (v/v) (Oxoid, Hampshire, UK) and was incubated at room temperature for 15 minutes. The amount of H2O2 was measured at 230 nm and the percentage inhibition of H2O2 scavenging activity was calculated using the same equation mentioned in (determination of radical –scavenging activity with DPPH assay section).
Determination of Metal Chelating activity
A mixture contains coffee sample, 2 mM FeCl2 (BDH, Poole, UK) and 5 mM ferrozine (Sigma-Aldrich, Poole, UK) was prepared and was incubated at room temperature for 10 minutes.11 The absorbance of the product was measured at 562 nm and the ferrozine-Fe2+ complex percentage inhibition was calculated following the equation described in (determination of radical –scavenging activity with DPPH assay section).
Determination of reducing power activity
To examine the ability of the water extracts of coffee samples to reduce Fe (III) to Fe (II),12 coffee extract samples were incubated in a water bath at 50˚C for 30 minutes with phosphate buffer (pH 6.6) and potassium ferricyanide (1%) (Sigma-Aldrich, Poole, UK). In order to acidify mixtures, 10% trichloroacetic acid (TCA) (Sigma-Aldrich, Poole, UK) was added to samples and were centrifuged at 6000 rpm for 10 minutes at 10˚C. Then, the supernatant was treated with deionized water and (1%) ferric chloride (Sigma-Aldrich, Poole, UK), and the final formed blue color product was measured at 700 nm.
Statistical Analysis
Data that were obtained from experiments were expressed as mean ± SEM. The readings were obtained from three independent experiments for each coffee sample. Results were analyzed by Graphpad prism version 7.01, using unpaired t test. Then the errors were corrected by Bonferroni’s test. The differences between means were considered statistically significant with P<0.05.
Results
The Selection of study Samples
In this study, an electronic survey was performed on 900 participants to identify the most consumed coffee types and brands in Jeddah, Saudi Arabia. The results from the survey revealed that six types of coffee, out of 11, were the most consumed coffee types. By classifying the results, according to roasting and processing properties, the six types were classified into two major types (ground and instant). The major three ground coffee types with higher consuming percentages were respectively, Turkish, Espresso, and Arabic (Ethiopian) coffee. Therefore, the Turkish coffee was selected as a representative sample for the ground coffee class. For the instant coffee, the results showed that the three major types with higher consuming percentages were respectively, Nescafé caffeinated red mug, Nescafé caffeinated gold and Nescafé decaffeinated gold. Hence, the Nescafé caffeinated red mug was chosen as a representative sample for the instant coffee type.
Identification of total polyphenol and flavonoid contents
Total polyphenol content was reported as Gallic acid equivalents/ serving size, whereas, the total flavonoid content was reported as catechin equivalents/ serving size. Table 1 represented the total contents of both polyphenol and flavonoid in the ground and instant coffees.
Table 1: Total polyphenol and flavonoid contents
| Sample | Total polyphenol contents
(µg Gallic Acid/serving size) |
Total flavonoid contents (µg Catechin/serving size) |
| Ground coffee | 845.77 | 765.6 |
| Instant coffee | 677.41 | 535.2 |
Determination of DPPH radical-scavenging activity
In this assay, the reduction in the absorbance at 516 nm, which can be recorded as a change from purple to yellow color, might indicate the ability of DPPH to scavenge free radicals by donating hydrogen. Results in figure 1 indicated that instant coffee had a higher radical scavenging ability (47%±1) compared to the ground coffee (27%±1.85) (P≤0.001).
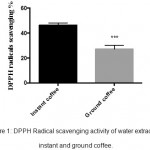 |
Figure 1: DPPH Radical scavenging activity of water extracts of instant and ground coffee.
|
Results are represented as mean ± SEM of three independent measurements (n=3). The data showed a significant difference in the radical scavenging activity according to the analysis performed by unpaired t test followed by a correction with Bonferroni’s test (***P≤0.001).
Determination of hydrogen peroxide scavenging activity
The antioxidants that present in coffee extracts, have the ability to scavenge H2O2 by donating electrons to water.13,14 Data in figure 2 revealed that instant coffee (73%±1.93) had much higher H2O2 scavenging ability than ground coffee (28%±1.78) (P≤0.001).
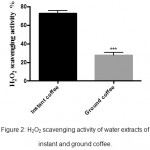 |
Figure 2: H2O2 scavenging activity of water extracts of instant and ground coffee.
|
Results are represented as mean ± SEM of three independent measurements (n=3). Data were obtained by unpaired t test and the errors were corrected with Bonferroni’s test (***P≤0.001).
Determination of Metal Chelating activity
Complexes can be formed when ferrozine reagent reacts with metals such as ferrous ion. These complexes have the ability to generate free radicals from peroxides and may lead to serious health complications.15 Therefore, chelating agents are important to capture ferrous ions before reacting with ferrozine. In contrast to the two previous assays (DPPH and H2O2 scavenging activities), the results of this assay indicated that ground coffee (52%±2.17) was significantly more active than instant coffee in chelating metals (36%±3.17) (P≤0.01) (figure 3).
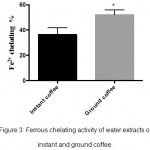 |
Figure 3: Ferrous chelating activity of water extracts of instant and ground coffee.
|
Results are represented as mean± SEM of three independent measurements (n=3). Data revealed that ground coffee has higher ability as metal chelator compared to instant coffee. The results were calculated by unpaired t test and the errors were corrected with Bonferroni’s test (P<0.05).
Determination of reducing power activity
This assay determines the ability of antioxidant compounds in sample extracts to reduce fe+3 to fe+2 by donating an electron, which in turn can act as an indicator for the intensity of antioxidant activity. Figure 4, showed an increase in the reduction ability of instant coffee compared to ground coffee extract (P≤0.05).
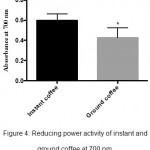 |
Figure 4: Reducing power activity of instant and ground coffee at 700 nm.
|
Results are illustrated as mean± SEM of four independent measurements (n=4). They were calculated by unpaired t test and the errors were corrected with Bonferroni’s test (*P≤0.05).
Results
Coffee is a rich source of antioxidants that help in fighting several diseases and improve the mood. Although ground and instant coffees are roasted and grounded, they are differing from each other in the brewing processing.5 Instant coffee is already brewed by manufacturers, which use various processes called agglomeration involves boiling, steeping, filtration and pressure to extract water-soluble compounds5. In contrast, ground coffee needs to brew immediately before consumption. However, the quality of the products varies depending on the coffee type (Robusta or Arabica) and the skills of roasting and brewing.16
In the current study, results showed significant difference in DPPH free radicals scavenging activity (instant coffee had higher radical scavenging ability than grounded coffee). Melanoidins, which were formed during roasting process in reaction called (Maillard reaction), are probably responsible for the low response of scavenging activity in ground coffee. Especially that most of ground coffees are prepared from beans with a dark roasted degree, which is higher in melanoidins fraction, while instant coffee is prepared from beans with a light medium roasted degree. Other studies performed on dark coffee brews, have found that these coffees have minimum antioxidant activity.17 Moreover, our results showed that percentage inhibition of instant coffee was significantly higher than ground coffee in H2O2 scavenging ability. The result of Long et al., was similar to our result.18 The brewing and roasting process of instant coffee in manufacturer might be better than home brewing of ground coffee particularly that some compounds of coffee are volatile and they might be evaporated during boiling process 5.
Interestingly, the ferrous ion-chelating assay performed on the two coffee samples showed that ground coffee had a significant increase in the chelating activity compared to instant coffee. Moreira et al., had the same result as our study.19 The content of active compounds (polyphenols and flavonoids) in ground coffee were higher and this may explain the better chelating activity (52%) compared to instant coffee (36%) (P<0.05). This means that there was a direct correlation between chelating ability and the content of active compounds.20 Some studies suggest that the melanoidins, which formed during roasting and, the amount of chlorogenic acid might be responsible for changing chelating activities.21
New researches are linking oxidative stress that resulted in chronic diseases development with antioxidant compounds. Accumulation of iron is found to be related to neurodegenerative diseases and aging processes and iron chelating agent is an efficient factor that prevents metals accumulation.22,23 The results from reducing power activity assay in this study showed that instant coffee was much active as a reducing agent than ground coffee. This result may explain the significant antioxidant activity of extracts from instant coffee.
In summary, in the present study, the antioxidant activity of the water extracts from two coffee types (ground & instant) which are commonly consumed in Jeddah, Saudi Arabia was assessed by using several colorimetric assays. Most assays showed that instant coffee had a higher antioxidant activity than ground coffee mainly as a scavenging and reducing agent. In contrast, ground coffee had stronger antioxidant activity especially as a metal chelating agent. The results of this study provided an overview of the effect of processing conditions specifically roasting degrees on the antioxidant activity.
Acknowledgements
This research was supported by the Science Research & Innovation Unit at the Faculty of Science, King Abdulaziz University in Jeddah, Saudi Arabia.
Footnotes
Authors’ Contribution
Ayat Badr Al-Ghafari developed the original idea, performed the statistical analysis and prepared the manuscript. Ghrob Hussein Althaqafi performed the experiments and prepared the manuscript.
References
- Hečimović I., Belščak-Cvitanović A., Horžić D., Komes D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011;129(3):991–1000.
CrossRef - Alves R., Casal S., Oliveira M. B. P. P. Tocopherols in coffee brews: Influence of coffee species, roast degree and brewing procedure. J Food Compos Anal. 2010;23(8):802–8.
CrossRef - Karakaya S., El S. N., Taş A. A. Antioxidant activity of some foods containing phenolic compounds. Int J Food Sci Nutr. 2001;52(6):501–8.
CrossRef - Mnatsakanyan M., Goodie T. A., Conlan X. A., Francis P. S., McDermott G. P., Barnett N. W., Shock D., Gritti F., Guiochon G., Shalliker R. A. High performance liquid chromatography with two simultaneous on-line antioxidant assays: Evaluation and comparison of espresso coffees. Talanta. 2010;81(3):837–42.
CrossRef - Chu Y. F. (ed) Coffee Constituents. Coffee: emerging health effects and disease prevention, 1st John Wiley & Sons, Inc. 2012;21–58.
- Gunduc N., El S. N. Assessing antioxidant activities of phenolic compounds of common turkish food and drinks on in vitro low-density lipoprotein oxidation. J Food Sci. 2003;68(8):2591-95.
CrossRef - Przygodzka M., Zielińska D., Ciesarová Z., Kukurová K., Zieliński H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. J Food Sci Technol. 2014;58(2):321–26.
CrossRef - Dewanto V., Wu X., Adom K. K., Liu R. H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010–14.
CrossRef - Bersuder P., Hole M., Smith G. Antioxidants from a heated histidine – glucose model system. I : Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J Amer Oil Chem Soc. 1998;75(2):181–87.
CrossRef - GὕlIN I., Alici H.A., Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull. 2005;53(3):281–85.
CrossRef - Končić M. Z., Barbarić M., Perković I., Zorc B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules. 2011;16(8):6232–42.
CrossRef - Karawita R., Siriwardhana N., Lee K. W., Heo M. S., Yeo I. K., Lee Y. D., Jeon Y. J. Reactive oxygen species scavenging, metal chelation, reducing power and lipid peroxidation inhibition properties of different solvent fractions from Hizikia fusiformis. Eur Food Res Technol. 2005;220(3):363–71.
CrossRef - Halliwell B., Clement M. V., Long L. H. Hydrogen peroxide in the human body. FEPS Lett. 2000;486(1):10–13.
CrossRef - Lee J., Koo N., Min D. B. Reactive oxygen species aging and antioxidative nutraceuticals. 2004;3(1):21–33.
CrossRef - Ebrahimzadeh M. A., Nabavi S. M., Nabavi S. F., Bahramian F., Bekhradnia A .R. Antioxidant and free radical scavenging activity of Officinalis L. Var. Angustifolius V. Odorata B. Hyrcana and C. Speciosum. Pak J Pharm Sci. 2010;23(1):29–34.
CrossRef - Murthy P. S., Naidu M. M. Sustainable management of coffee industry by-products and value addition—A review. Resour Conserv Recycl. 2012;66:45–58.
CrossRef - Delgado-Andrade C., Ruiàn-Henares J. A., Morales F. J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J Agric Food Chem. 2005;53(20):7832–36.
CrossRef - Long L. H., Lan A. N., Hsuan F. T., Halliwell B. Generation of hydrogen peroxide by antioxidant beverages and the effect of milk addition. Is cocoa the best beverage? Free Rad Res. 1999;31(1):67–71.
CrossRef - Moreira D. P., Monteiro M. C., Ribeiro-Alves M., Donangelo C. M., Trugo L. Contribution of chlorogenic acids to the iron-reducing activity of coffee beverages. J Agric Food Chem. 2005;53(5):1399–1402.
CrossRef - Ebrahimzadeh M. A., Pourmorad F., Bekhradnia A. R. Iron chelating activity phenol and flavonoid content of some medicinal plants from Iran. J. Biotechnol. 2008;7(18):3188–92.
CrossRef - Takenaka M., Sato N., Asakawa H., Wen X., Murata M., Homma S. Characterization of a metal-chelating substance in coffee. Biosci Biotechnol Biochem. 2005;69(1):26–30.
CrossRef - Oboh H. A., Omoregie I. P. Total phenolics and antioxidant capacity of some Nigerian beverages. Nig J Basic Appl Sci. 2011;19(1):68–75.
CrossRef - Yardim Y. Electrochemical behavior of chlorogenic acid at a boron-doped diamond electrode and estimation of the antioxidant capacity in the coffee samples based on its oxidation peak. J Food Sci. 2012;77(4):408–13.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





