Manuscript accepted on : 14 March 2017
Published online on: --
Plagiarism Check: Yes
Farah J. Hashim1, Suaad M. Hussain2 and Muayad S. Shawkat1
1Department of Biotechnology /College of Science/ University of Baghdad/ Baghdad/ Iraq.
2Department of Chemistry /College of Science/ University of Baghdad/ Baghdad/ Iraq.
Corresponding Author E-mail: farah_bio_2020@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2412
ABSTRACT: Coumarin is a phenolic natural product extracted from yellow clover Melilotus officinalis by maceration in ethanol solvent. Crude extract was analyzed by high performance liquid chromatography then divided into two portions, first one characterized by Fourier transform infrared spectroscopy (FTIR) and investigated for anticoagulation activity by prothrombin time (PT) while the second portion subjected for fractionation using column chromatography (CG) and fractions were characterized by FTIR, each fraction was detected as anticoagulant agent. As results, coumarin crude extract eluted with retention time (7.6 min.) as standard coumarin by HPLC, molecular spectrums of fractions obtained by column chromatography were measured using FTIR. Anticoagulation activity of crude extract results were dose dependent pattern .We concluded that M. officinalis is a good source for coumarin and coumarin derivatives as shown in (HPLC) analysis while using column chromatography (CG) and (FTIR) considered as valuable techniques for separation and characterization .
KEYWORDS: Anticoagulant; column chromatography coumarin; FTIR; HPLC; Melilotus officinalis;
Download this article as:| Copy the following to cite this article: Hashim F. J, Hussain S. M, Shawkat M. S. Separation, Characterization and Anticoagulant Activity of Coumarin and its Derivatives Extracted from Melilotus officinalis. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Hashim F. J, Hussain S. M, Shawkat M. S. Separation, Characterization and Anticoagulant Activity of Coumarin and its Derivatives Extracted from Melilotus officinalis. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22719 |
Introduction
Many years ago, plants were consumed for medicinal purposes which using as herbal or alternative homemade remedies, either as extracts, or as purified potential fractions with biological activities. The world health organization (WHO) establishes that 80% of human beings in growing counties (65% of the world’s population) remain confidences in using bioactive derivatives utilized from plants (Karakas et al., 2012). M. officinalis (yellow sweet clover) is being as one of important therapeutic plants which were utilized since long times ago by several herbalists and physicians as a digestive, eye inflammation, hemorrhoids, stomach ulcer and disorders. Extract of dried petals of M. officinalis took part in pharmaceutical effects for the symptomatic treatment of venous-lymphatic in-sufficiency (Mahajan and Gajare, 2012). Coumarin considered as the major active compound in the M. officinalis crude extract and appear to function in various capacities in different plant defense mechanisms against insect herbivores and fungi (Murray et al., 1982) , in addition, this component has biological properties due to obstacle blood clot formation in the vessel (Klein, 2011). In this study, two techniques were used in analysing and detecting the presence of coumarin compound in crude extract ; High performance liquid chromatography technique (HPLC) by pass a pressurized liquid “referred to mobile phase” and a sample mixture through out a column contain adsorbent particles, causing the separation of the sample compounds because they have various degrees of interaction with the absorbent; while the other technique was Fourier Transform Infrared Spectroscopy (FTIR) briefly by a moving mirror inside the apparatus changes the distribution of infrared light that passes through the interferometer. The signal directly recorded, named an “interferogram”, represents light output as a function of mirror position. An apparatus records this raw data into the final result. Anti-coagulant activity measured by prothrombin time kit (PT).
Material and Methods
Preparation of Plant Extract
Leaves of M. officinalis were collected from gardens of Baghdad University, (10) gm of dried powdered plant sample defatted with (100) ml hexan for (3) hours. Then extracted in (100) ml of ethanol by maceration and crude extract evaporated at 40°C by using incubator and resultant was collected and stored at 4°C until use (Szewczyk and Koka, 2012).
Detection by High Performance Liquid Chromotochraphy Analysis
Chromatographic separation of coumarins was performed by apparatus Shimadzu LC-10 system with LC-10AD pump, UV-variable wave length detector (at 203 nm) . Sample passes through a column C-18, (5μm, 250 mm x 0.6 mm). Separations were carried out with isocratic mode, acetonitrile: water (40:60; v/v) utilized as a mobile phase at a flow rate of (1) mL min-1; with an injection volume of 20 μL, The HPLC system was operated at room temperature (23 ± 10C). The sample was injected with concentration 10mg / 1 ml while standard coumarin as 1mg/ml (Celeghini, et al., 2001).
Separation of Coumarin Derivatives by Column Chromatography
Separated active ingredients were added as three volumes of ether to one volume of crude extract in separating funnel then shaked very well , two layers obtained as upper ether layer and lower aqueous layer.
Column (3.1 x5) packed with suspension of silica gel and methanol, after the stability of the gel the column were washed with methanol then added ether layer to the column. Washed with solvent system consist of diethyl ether with 10% acetic acid. Fractions were collected as 1 ml for each tube with flow rate 12ml/hr.
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
Chemicals that used in this study were purchased from Merck, BDH, Sigma Aldrich and Fluka companies. Melting point have been estimated using digital stuart scientific SMP3 melting point apparatus. FTIR spectra were established by Shimadzu FTIR- 8400 Fourier transform infrared spectrophotometer using KBr discs in the (4000-600) cm-1 spectral range (Sliverstien and Webster, 1998).
Prothrombin Time Assay Procedure
A test tube contains 0.1 ml of plasma was kept in incubator for 3 to 5 min at 37°C after that 0.2 ml of Biolabo reagent (biolabo SA, France) was added to the test tube after warming the reagent at 37°C. Vary concentrations of M. officinalis coumarin extract were added separately to the test tube, the coagulation time or prothrombin time (PT) was estimated in seconds by stopwatch in addition to recording coagulation time of +ve and –ve control. Each test concentration was repeated three times and data average used. Normal saline used instead of extract in –ve control while EDTA as a +ve control (Govindappa et al., 2015
Results and Discussion
HPLC Analysis
officinalis extract was analyzed by HPLC apparatus and coumarin compounds eluted retention time at 7.66 min. which were closed to retention time of Standard coumarin which appeared at 7.63 min. (Fig. 1 a & b).
As showed in (Fig.1 a), the concentration of coumarin components in the plant extract is low due to small peak of eluted compounds compared with high peak of standard coumarin in (Fig.1 b) although the concentration used of M. officinalis extract was 10 mg/ml while the concentration standard coumarin prepared with 1mg/ml.
 |
Figure 1: a/b Fourier transform infrared spectroscopy (FTIR) characterization of crude extract: |
M. officinalis crude extract confirmed the presence of phenolic and amino coumarin derivatives (7-hydroxy coumarin and 6-amino coumarin) were afforded a pale deep green color and m.p (216-236 0C) also phenolic test and amino group test have confirmed the presence of phenol and amino groups (Ralph etal., 2004). FTIR spectrum of these compounds indicate that Ѵ– H: (3375) cm-1 of hydroxyl group while a Ѵ- NH2: asym. (3375) cm-1 and sym. (3193) cm-1 , also the presence of ѴC-H aromatic at (3030) cm-1 , Ѵ C=O 🙁 1737) cm -1 of carbonyl group, Ѵ C=C : (1622) cm-1 of olefenic group and Ѵ C-O-C at (1072) cm-1 (Silverstein et al., 2005); FTIR spectral data crude extract coumarin shown by figure (2)
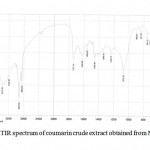 |
Figure 2: FTIR spectrum of coumarin crude extract obtained from Melilotus officinalis:
|
M. officinalis fractionated by column chromatography separation, collected and subjected for FTIR apparatus and the results are presents as following:
FTIR spectrum of 3,3-methylene bis 4-hydroxy-coumarin (dicoumarol) compounds indicate that ѴO – H : (3645) cm-1 while ѴC-H aromatic at (3097) cm-1 , Ѵ C – H aliphatic ( 2958 , 2927) cm -1, Ѵ C=O : (1731) cm-1 and Ѵ C=C aromatic at (1568, 1550) cm-1 in addition to presence ѴC-O-C (asym. 1128, sym 1072) cm-1 (REF); FTIR spectral data by figure (3).
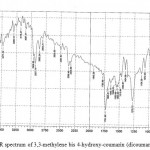 |
Figure 3: FTIR spectrum of 3,3-methylene bis 4-hydroxy-coumarin (dicoumarol) compounds
|
FTIR spectrum of 2-hydroxy-4-methylene-5-oxo-2, 3- dihydro furane [2-3a][1] benzo pyrane compounds indicate that ѴO – H : (3365) cm-1 while ѴC-H aromatic at (3076) cm-1 , Ѵ C – H alphatic ( 2933) cm -1 and Ѵ C=C of olefien at (1625) cm-1 in addition to presence Ѵ C=C aromatic (1577, 1425) cm-1 and Ѵ C-O ( 1074) cm-1 (REF); FTIR spectral data by figure (4).
![Figure 4: FTIR spectrum of 2-hydroxy-4-methylene-5-oxo-2, 3- dihydro furane [2-3a][1] benzo pyrane compounds](https://www.biotech-asia.org/wp-content/uploads/2017/03/Vol_14_no1_Sepa_Fara_fig4-150x150.jpg) |
Figure 4: FTIR spectrum of 2-hydroxy-4-methylene-5-oxo-2, 3- dihydro furane [2-3a][1] benzo pyrane compounds
|
FTIR spectrum of 2-hydroxy-5-methylene-6-oxo-2 H,6H 3,4 dihydro pyronal [3,2-C][1] benzo pyranone compounds indicate that ѴO – H : (3429) cm-1 while ѴC-H aromatic at (3001) cm-1 , Ѵ C – H alphatic ( 2945) cm -1, Ѵ C=O : (1699) cm-1 and Ѵ C=C at (1641) cm-1 in addition to presence Ѵ C=C aromatic (1562)cm-1 and Ѵ C-O ( 1020) cm-1 (REF); FTIR spectral data by figure (5).
![Figure 5: FTIR spectrum of 2-hydroxy-5-methylene-6-oxo-2 H,6H 3,4 dihydro pyronal [3,2-C][1] benzo pyranone compounds](https://www.biotech-asia.org/wp-content/uploads/2017/03/Vol_14_no1_Sepa_Fara_fig5-150x150.jpg) |
Figure 5: FTIR spectrum of 2-hydroxy-5-methylene-6-oxo-2 H,6H 3,4 dihydro pyronal [3,2-C][1] benzo pyranone compounds
|
FTIR spectrum of 2-hydroxy-5-oxo-3,4 dihydro furan [3-2 a][1] benzo pynone compounds indicate that ѴO – H : (3390) cm-1 while ѴC-H aromatic at (3015) cm-1 , Ѵ C – H alphatic ( 2927) cm -1, Ѵ C=O : (1733) cm-1 and Ѵ C=C aromatic at (1573, 1614) cm-1 in addition to presence ѴC-O-C (asym. 1118)cm-1(sym. 1074) cm-1 (REF); FTIR spectral data by figure (6).
![Figure 6: FTIR spectrum of 2-hydroxy-5-oxo-3,4 dihydro furan [3-2 a][1] benzo pynone compounds](https://www.biotech-asia.org/wp-content/uploads/2017/03/Vol_14_no1_Sepa_Fara_fig6-150x150.jpg) |
Figure 6: FTIR spectrum of 2-hydroxy-5-oxo-3,4 dihydro furan [3-2 a][1] benzo pynone compounds
|
FTIR spectrum of 2-hydroxy-5-oxo-3,4 dihydro pyronal [3,2-C][1] benzopyrane compounds indicate that ѴO – H : (3479) cm-1 while ѴC-H aromatic at (3045) cm-1 , Ѵ C – H alphatic ( 2921) cm -1, Ѵ C=O : (1735) cm-1 and Ѵ C=C aromatic at (1581, 1461) cm-1 in addition to presence ѴC-O ( 1099) cm-1 (REF); FTIR spectral data by figure (7).
![Figure 7: FTIR spectrum of 2-hydroxy-5-oxo-3,4 dihydro pyronal [3,2-C][1] benzopyrane compounds](https://www.biotech-asia.org/wp-content/uploads/2017/03/Vol_14_no1_Sepa_Fara_fig7-150x150.jpg) |
Figure 7: FTIR spectrum of 2-hydroxy-5-oxo-3,4 dihydro pyronal [3,2-C][1] benzopyrane compounds
|
FTIR spectrum of 6-amino coumarin compounds indicate that Ѵ NH2 (asym. 3415, sym. 3390) cm-1 while ѴC-H aromatic at (3087) cm-1 , Ѵ C=O : (1731) cm-1 and Ѵ C=C aromatic at (1622, 1544, 1461) cm-1 in addition to presence ѴC-O(1122)cm-1 (REF); FTIR spectral data by figure (8).
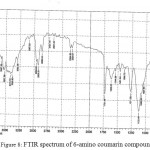 |
Figure 8: FTIR spectrum of 6-amino coumarin compounds
|
FTIR spectrum of mixture consist of 6-amino coumarin and 6-cyano coumarin compounds indicate that ѴC-H aromatic at (3051) cm-1 , Ѵ C = ( 2360) cm -1, Ѵ C=O : (1737) cm-1 and Ѵ C=C aromatic at (1625, 1595, 1575) cm-1 in addition to presence ѴC-O (1097)cm-1 (REF); FTIR spectral data by figure (9).
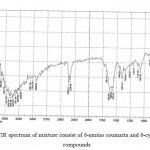 |
Figure 9: FTIR spectrum of mixture consist of 6-amino coumarin and 6-cyano coumarin compounds
|
FTIR spectrum of 6-carboxylic coumarin compounds indicate that ѴO – H : (3452-2896) cm-1 while ѴC-H aromatic at (3080) cm-1 , Ѵ C=O : (1718) cm-1 and Ѵ C=C aromatic at (1577) cm-1 in addition to presence ѴC-O (1076) cm-1 (REF); FTIR spectral data by figure (10).
 |
Figure 10: FTIR spectrum of 6-carboxylic coumarin compounds
|
FTIR spectrum of mixture contain 7-acetyl coumarin and 4-methyl-2,5-dioxo-3-phenyl-2H,5H-pyrano-[3,2-c][l]- Benzopyran compounds indicate that ѴC-H aromatic at (3004) cm-1 , Ѵ C – H alphatic ( 2987) cm -1, Ѵ C=O : (1735) cm-1 and Ѵ C=C aromatic at (1577, 1514) cm-1 in addition to presence ѴC-O (1043) cm-1 (REF); FTIR spectral data by figure (11).
![Figure 11: FTIR spectrum of mixture contain 7-acetyl coumarin and 4-methyl-2,5-dioxo-3-phenyl-2H,5H-pyrano-[3,2-c][l]- Benzopyran compounds](https://www.biotech-asia.org/wp-content/uploads/2017/03/Vol_14_no1_Sepa_Fara_fig11-150x150.jpg) |
Figure 11: FTIR spectrum of mixture contain 7-acetyl coumarin and 4-methyl-2,5-dioxo-3-phenyl-2H,5H-pyrano-[3,2-c][l]- Benzopyran compounds
|
FTIR spectrum of 4-hydroxy coumarin compounds indicate that ѴO – H : (3382) cm-1 while ѴC-H aromatic at (3091) cm-1 , Ѵ C=O : (1726) cm-1 and Ѵ C=C aromatic at (1625, 1552) cm-1 in addition to presence ѴC-O-C (1074)cm-1 (REF); FTIR spectral data by figure (12).
 |
Figure 12: FTIR spectrum of 4-hydroxy coumarin compounds
|
Anti-Coagulant Activity
In this study, anti-coagulant activity of the crude extract was investigated by prothrombin time kit and results were showed by table (1) below:-
Table 1: Anticoagulant activity of M. officinalis crude extract
| sample | concentrations | Prothrombin time/ sec. |
| M. officinalis Crude extract | 25 µl | 80 Sec. |
| 50 µl | 140 Sec. | |
| 75 µl | No-clot | |
| -ve control | 100 µl/ normal saline | 30 Sec. |
| +ve control (EDTA) | 100 µl | 3100 |
Prothrombin time of treated blood (46 Sec., 140 Sec. and No-clot) with different concentration of M. officinalis extract (25, 50 and 75 µl) respectively, showed anti-coagulation activity by dose-dependent manner compared with the negative control which was 14 seconds while treated blood with EDTA as +ve control prevent clot formation.
Normally, blood should be stay as a fluid inside the vascular system while forming clot formation directly when the vascular exposing to injury or accident. The predominant agents for keeping blood fluidity such as parenteral anti-coagulant heparin and its derivatives promote a natural inhibitor of coagulant protease.
Fibrinolysis causing activate fluidity of blood due to lyse intravascular thrombi, increasing the stimulation of fibrinolysis may cause systemic destroying of fibrinogen and coagulation factors so minimal concentration of coumarin involved in ethanolic extract of M. officinalis (sweet clover) block multiple steps of the coagulation cascade by being competitive inhibitors of Vitamin-K in the biosynthesis of prothrombin (Jain and Jos, 2012).
Anti-Coagulant Activity of Coumarin Derivatives fractions
officinalis crude extract fractionations by column chromatography were tested as anti-coagulant agents and the results shown by table (2):-
Table 2: Anticoagulant activity of Coumarin and coumarin derivatives separated from M. officinalis extract by column chromotochraphy
| Sample | Concentrations/µl | Prothrombin time (PT)/Sec. |
| I. 3,3-methylene bis 4-hydroxy-coumarin (dicoumarol) | 25 | 200 |
| 50 | 350 | |
| 75 | 2800 | |
| II. 2-hydroxy-4-methylene-5-oxo-2, 3- dihydro furane [2-3a][1] benzo pyrane | 25 | 110 |
| 50 | 150 | |
| 75 | 2000 | |
| III. 2-hydroxy-5-methylene-6-oxo-2 H,6H 3,4 dihydro pyronal [3,2-C][1] benzo pyranone | 25 | 190 |
| 50 | 210 | |
| 75 | 1150 | |
| IV. 2-hydroxy-5-oxo-3,4 dihydro furan [3-2 a][1] benzo pynone | 25 | 140 |
| 50 | 220 | |
| 75 | 1800 | |
| V. 2-hydroxy-5-oxo-3,4 dihydro pyronal [3,2-C][1] benzopyrane | 25 | 95 |
| 50 | 150 | |
| 75 | 950 | |
| VI. 6-amino coumarin | 25 | 80 |
| 50 | 120 | |
| 75 | 935 | |
| VII. mixture of 6-amino coumarin and 6-cyano coumarin | 25 | 75 |
| 50 | 100 | |
| 75 | 890 | |
| VIII. 6-carboxylic coumarin | 25 | 60 |
| 50 | 90 | |
| 75 | 875 | |
| IX. 4-hydroxy coumarin | 25 | 60 |
| 50 | 80 | |
| 75 | 800 |
The results indicated that Dicourmal or bis 4-hydroxy coumarin gave the higher anti-coagulant activity with (2800 sec.) due to presence of methoxyl group so the modification of 4-hydroxy coumarin by adding methoxyl group will increase anticoagulation effect (Venugopala, et al., 2013& Mathur and Arora, 1963). dicumarol, inhibits the clotting mechanism by interaction the production process of an important factors which were produced by liver and necessary for vitamin K activity (Asif, 2015).
2-hydroxy-4-methylene-5-oxo-2, 3- dihydro furane [2-3a][1] benzo pyrene compound and 2-hydroxy-5-methylene-6-oxo-2 H,6H 3,4 dihydro pyronal [3,2-C][1] benzo pyranone compound showed PT with (2000 and 1150 Sec.) respectively, Chmielewska & Cieglak (1958) analyzed the results of these compounds activity according to structural similarity with vitamin K, while 2-hydroxy-5-oxo-3,4 dihydro furan [3-2 a][1] benzo pynone compound(1800 Sec.) or 2-hydroxy-5-oxo-3,4 dihydro pyronal [3,2-C][1] benzopyrane (950 Sec.) which were considered as anti-vitamin K due to structure contains cyclic hemiacetals.
Present data shows that the following compounds (6-amino coumarin, mixture of 6-amino coumarin and 6-cyano coumarin, and 6-carboxylic coumarin ) compounds gave valuable activity with time as (935, 890 and 875 Sec. respectively) due to presence of nitrogen, cyano and carboxyl substituents in 6- position.
Mixture of 7-acetyl coumarin and 4-methyl-2,5-dioxo-3-phenyl-2H,5H-pyrano-[3,2-c][l]- Benzopyran result data have a considerable example mention relation between activity and molecular shape refer to extra ring which were added to 4-hydroxy system Otherwise, 4-hydroxy coumarin have activity against clots as (800 sec.), support Link (1943)hypothesis about minimum structure required for anticoagulation.
Coumarin derivatives consuming orally is effective treatment for a wide range of clot dependent disorders. Investigations have shown that oral consuming treatment is medicinally reactive against intravascular clot formation in patients suffering from thromboembolism and atrial fibrillation disorders in blood stream; this is prevention stroke and mortality in cases of operations of myocardial and heart valves. Otherwise, anticoagulant medications using with cases suffering from heart disorders dependent rheumatism due to inhibiting variety proteins causing activation clot formation. Continuously treated with heparin causing osteoporosis beside uncomfortable situation for the patients so the coumarins are used instead of heparin (Goodman & Gilman’s, 2006).
Conclusion
Coumarins naturally found in M. officinalis consider as important phenolic compounds due to their clinically and biologically applications. HPLC proved as a useful tool for quality detection while FTIR is valuable for control molecular characterization of these compounds. Coumarins shows significant anticoagulant potential, especially purified coumarin derivatives compounds.
Acknowledgements
We sincerely thank laboratory of Analytical chemistry and laboratory of central environmental in college of science for their cooperation.
Conflict of Interest
The authors declare no conflict of interest associated with this work.
References
- Karakas F .,Yildirm A and Turker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities .TUBITAK. 2012;10:641-652.
- Mahajan R and Gajare S. Manifestation erectile dysfunction with adaptogenic antioxidant aphrodisiac plants. International Journal of Pharmaceutical and Biomedical research. 2012;3(1):52-68.
- Klein H . Yellow Sweet Clover Melilotus officinalis(L.) lam . Alaska Natural Heritag program , University of Anchorag 2011;2-8.
- Szewczyk K and Koka A.,Dr. Rao V (Ed). Analytical methods for Isolation Separation and Identification of furanocoumarin in plant materials Phytochemicals-A global prespective of their role in nutrition and healt. INTECH. 2012;58-92.
- Renata M. S., Janete C., Vilegas H. Y and Lanças F. M. Extraction and Quantitative HPLC Analysis of Coumarin in Hydroalcoholic Extracts of Mikania glomerata (“guaco”) Leaves. J. Braz. Chem. Soc. 2001;12(6):706-709.
- Arora R. B and Mathur C. N. Relationship between structure and anticoagulant activity of coumarin derivatives. Brit .J. Pharmcol. 1962;20:29-35.
- Murray R. D. H.,Mendez J and Brown S. A. Coumarin activity in plants and bioorganism aspects. John Wiley. 1982;2:45-55.
- Silverstein R., webster F and kiemle D. spectroscopic identification of organic compound. 7th John wiley & sons, Inc. USA. 2005.
- Venugopala K. N., Rashmi V and Odhav B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Research International. 2013:1-14. Article ID 963248.
- Asif M. Pharmacologically potentials of different substituted coumarin derivatives c. 2015;1(1):1-11.
- Jain P. K and Joshi H. Coumarin: Chemical and Pharmacological Profile. Journal of Applied Pharmaceutical Science. 2012;2:6:236-240.
- Govindappa M.,Naik C., Prakash B and Channabasava. Anticoagulant activity of partially purified coumarin(s) extracts of Sonchus oleraceus. Advancement in Medicinal Plant Research. 2015;3(3):87-91.
- Sliverstien R. M and Webster X. Spectroscopic identification of organic compounds. Sixth edition. John wiely and Sons. New York. 1998.
- Goodman & Gilman’s. The Pharmacological basis of therapeutics: Blood coagulation and Anti-coagulant, thrombolytic and Anti-platelet drugs. 11th ed. 2006;1325-1328.15.
- Link K. P. The anticoagulant from sweet clover hay. Harvey Lect., 39, 1943, 162-216.

This work is licensed under a Creative Commons Attribution 4.0 International License.





