Manuscript accepted on : 12 May 2016
Published online on: 28-06-2016
Shamsu Adnan Muhammad1 and S. M. Abubakar2
1Department of Biotechnology, SRM University, Kattankulathur, Chennai, India 2Department of Biochemistry,Bayero University, Kano, Nigeria Correspondence Author Email: samgwarzo0113@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2153
ABSTRACT: The phytochemicals in aqueous extract of Chrysophyllumalbidum seeds kernel were determined qualitatively and quantitatively in this study with a view to assess the potentials of this kernels as food and medicine. Results of this research showed that the seed kernel extracts contained alkaloids, flavonoids, tannins, cardiac glycosides and saponins. Quantitatively, C.albidum seed kernelwas found to contain alkaloids (3.56g/100g),tannins (2.184g/100g), cardiac glycosides (1.88g/100g), saponins (0.24g/100g) and flavonoids (0.15g/100g). Thepresences of these phytochemicals suggest the potentials of the kernel of C.albidum for use as a neutraceutical.
KEYWORDS: Chrysophyllumalbidum; alkaloids; flavonoids; tannins; cardiac glycosides; saponins; terpenes; steroids; anthraquinones
Download this article as:| Copy the following to cite this article: Muhammad S. A, Abubakar S. M. Qualitative and Quantitative Determination of Phytochemicals In Aqueous Extract of Chrysophyllumalbidum Seed Kernel. Biosci Biotech Res Asia 2016;13(2). |
| Copy the following to cite this URL: Muhammad S. A, Abubakar S .M. Qualitative and Quantitative Determination of Phytochemicals In Aqueous Extract of Chrysophyllumalbidum Seed Kernel.. Biosci Biotech Res Asia 2016;13(2). Available from: https://www.biotech-asia.org/?p=14064 |
Introduction
Plants that possess therapeutic properties or exert beneficial pharmacological effects on the animal body are generally designated as “medicinal plant” [1]. It’s now been established that the plants which naturally synthesize and accumulate some secondary metabolites, like alkaloids, tannins, glycosides, volatiles oil and contain minerals and vitamins, possess medicinal properties[1].
Medicinal plants constitute an important natural wealth of a country. They play a significant role in providing primary health care services to rural people. They serve as therapeutic agents as well as important raw materials for the manufacture of traditional and modern medicines. Substantial amount of foreign exchange can be earned by exporting medicinal plants to other country. In this way indigenous medicinal plants play a significant role in the economy of a country[2].
The practice of traditional medicine is worldwide custom and has preoccupied mankind in most civilized country [2]. In Nigeria, both herbal traditional and orthodox medicines are practiced, through the conventionally trained medical doctors are contemplating the former. Despite the fact that traditional approach to health care delivery system is popularly practiced and in spite of the availability of medicinal herbs in our local market, there are very few if any, in government health centers. This could be due to lack of adequate information on the composition, processing, dosage and crude of the medicinal plant preparation [1].
The ChrysophyllumalbidumG.DonHoll(sapotaceae) tree is common throughout the tropical central, east and West African regions for its sweet edible fruits and various ethnomedicinal uses[3, 4].Chrysophyllumalbidum fruits (known as African star apples) are widely consumed in southern Nigeria. The fruit is seasonal (December-March), ovoid to sub-globose, pointed at the apex, is orange to golden yellow when ripe and the pulp within the peel may be orange, pinkish, or light yellow. The pulp contains three to five seeds which are not usually eaten [1]. The seeds-coats are hard, bony, shiny, and dark brown, and when broken reveal white-colouredcotyledons.The fleshy fruit pulp is suitable for jams and is eaten especially as snack by both young and old [4, 5].The fruit has been found to have the highest content of ascorbic acid per 100g of edible fruit or about 100 times that of oranges and 10 times of that of guava or cashew [6]. It is reported as an excellent source of vitamins, irons, flavours to diets [7, 8].
In addition, its seeds are a source of oil, which is used for diverse purposes. The fruits also contain 90% anacardic acid, which is used industrially in protecting wood and as a source of resin, while several other components of the tree including the roots and leaves are used for medicinal purposes[9]. The bark is used as a remedy for yellow fever and malaria, while the leaves are used as emollients and for the treatment of skin eruptions, diarrhea and stomach-ache, which are as a result of infections and inflammatory reactions [9].
It is rich sources of natural antioxidant and have been established to promote health by acting against oxidative stress related disease such infections as; diabetics, cancer and coronary heart diseases[10].
Recently studies have shown a diminished risk of chronic diseases in populations consuming diets high in fruits vegetables and it has been suggested that antioxidants found in large quantities in fruits and vegetables may be responsible for this protective effect[11].Generally, foods antioxidant acts as reducing agents, reversing oxidation by donating electrons and hydrogen ions. Much attention has been focused on natural antioxidants and some antioxidants isolated from natural sources with high activity have been reported[12].
Materials and Methods
Sample Collection
The Chrysophyllumalbidum seed kernel were obtained from the Rimi Market Kano and identified at the department of biological science Bayero University Kano, Nigeria. The pericarp and mesocarp region of the Chrysophyllumalbidumfruit were isolatedfrom the endocarp region (seed) and then dried for weeks.
Preparationand extraction of the aqueous extracts of C. albidum seeds kernel
The outer shells of the seeds were cracked, open and the inner soft parts removed and dried at room temperature for a period of 3 days and afterwards grounded into powder. The powdered seeds were sieved and stored properly. The aqueous extract of C.albidum seeds kernel was prepared by mixing 50g of the powder in 250g of distilled water and then stored for 24 hours [13].
Methods
Qualitative Phytochemical Screening
Ferric chloridetest for Tannins
Aportion of the sample (3g) was boiled in 50cm3 of distilled water for 3 minutes on the heating mantle. The mixture is filtered and the resulting filtrate was used to carry out test for tannins below.
A portion of the liquid extract above was diluted with distilled water in a ratio of 1:4 and a few 2 drops of 10% ferric chloride were added. A blue or green colour indicates the presence of tannin[14].
Test for Saponins (Froth test)
To a small portion of powdered sample 95% ethanol was added and boiled. The mixture was filtered and 2.5cm3 of the filtrate was added to 10cm3 of distilled water in a test tube. The test tube was stopped and shaken vigorously for about 30second; it was allowed to stand for over 30minutes. Honey-comb froth is indicative of the presence of saponin [2].
Test for Flavonoids
A quantity (5g) of the powdered sample was completely dissolved in acetone. The residue is extracted in warm water after evaporating the acetone on a water bath. The mixture was filtered and the filtrate is used for the following test.
Sodium hydroxide test for Flavonoids
To (5cm3) of 10% sodium hydroxide equal volume of the detanned water extracts of C.albidumwas added. A yellow solution indicates the presence of flavonoids [2].
Ferric chloride test for Flavonoids
A portion (2cm3) of extract was diluted with distilled water in a ratio of 1:4 and a few 3 drops of 10% ferric chloride (FeCl3) solution is added. A green or blue solution indicates the presence of flavonoids [2].
Test for Alkaloids
An extract of the powdered sample was prepared by macerating 3g of the powdered sample in 50cm3 of methanol. The extract was evaporated to dryness, 0.5g of the extract was mixed with 5cm3 of 1% aqueous hydrochloric acid and 1cm3 of the filtrate is treated with a few drops of Hagar’s reagent. Turbidity or precipitation with the reagent is taken as evidence for the presence of alkaloids in the extract[15].
Test for Terpenes
The plant extract was dissolved in 2cm3 chloroform, 1cm3 each of acetic anhydride and concentrated H2SO4 were added by the side of the test tube. A reddish-violet colour indicates the presence of terpenes [2].
Test for Anthraquinones
A portion of the powdered sample (0.5g) was taken in test tube and 10cm3 of chloroform was added and shaken for 5minutes. The extract was filtered and equal volume of ammonia was added and shaken. A bright pink colour in the upper aqueous layer indicates the presence of anthraquinones [14].
Test for Steroids
Concentrated H2SO4 (1cm3) was added to 1cm3 of test extract. A red colour indicates the presence of steroidal ring[17].
Test for cardiac glycosides
2.0cm3 of the ethanol extract was taken into a test tube and evaporated to dryness. The residue was dissolved in acetic anhydride and chloroform was then added. By means of a pipette concentrated sulphuric acid was added by the side of the tube. A brownish ring at the interface of the two liquids and the appearance of violet colour in the supernatant layer would indicate the presence of cardiac glycosides as reported by [2].
PhytochemicalQuantification Analysis (El-Olemyet al., 1994)
Determination of Alkaloids
To a 10ml extract of C.albidumkernel in 250ml separatory funnel followed by 5ml dil. H2SO4 and 5ml of distilled water were added. The extract was shaken twice with 10ml CHCl3 and the combined CHCl3 extract was transferred to a second separating funnel containing 5ml dilute H2SO4 and 10ml of distilled water. The CHCl3 layer was discarded and the aqueous acidic layer was transferred to the contents of the first separating funnel. The extract was made alkaline with ammonia and shaken for about half a minute.
The extract alkaloid was extracted completely by successive portion of CHCl3 each of 20ml. (complete extraction was tested using Mayer’s reagent). The combined CHCl3 extract was shaken with 5ml distilled water. The extract was run through a plug of cotton wool previously muster with CHCl3 and covered with a little anhydrous sodium sulphate, the sodium sulphate was washed with 5ml of CHCl3. The combined CHCl3 extract was received into a 250ml dry conical flask.
The CHCl3 was completely distilled and 5ml neutral alcohol was added and evaporated on a boiling water bath. The residue was further heated on the boiling water bath for about 10 – 15 minute (to remove volatile bases). The residue was dissolved in 2ml of CHCl3, and 20ml of N/50 H2SO4 was added and warmedon a water bath to remove the CHCl3completely and cooled. The excess and was titrated with N/50 NaOH using methyl red as indicator till the first drop of N/50 NaOH caused color change from pink to yellow
Calculation
1ml of N/50 NaOH ≡ 0.005787g alkaloids
%Alkaloids content = [20ml taken of N/50H2SO4 ] x 0.005787 x 100/10
Determination of Flavonoids
A 5ml extract was transferred to a small flask and then hydrolysed by heating on a water bath with 10ml of 10% H2SO4 for 30minutes. The original volume was reduced to half and the mixture was cooled on ice for 15minutes where the flavonoids are pre-evaporated.
The cooled solution was filtered; the residue was dissolved by pouring 50ml of warm 95% ethanol and further made to 100ml with 95% ethanol. A 5ml aliquot was pipetted into a 25ml volumetric flask and diluted to volume with 50% ethanol. The absorbance of the resulting solution was measured at 370nm against 50% ethanol blank. Flavonoid concentration was calculated using a reference curve of pure quercetin.
Determination of Tannins
5ml of plant extract was transferred to a stoppered conical flask and 25ml of 0.1N iodine and 10ml of 4% NaOH were added. This was mixed and kept in the dark for 15minutes. The mixture was diluted with water and acidified with 4% H2SO4 (10ml). The mixture was titrated with 0.1N sodium thiosulphate solution using starch solution as indicator. The number of ml of 0.1N Na2S2O3 used corresponds to the sum of tannins and pseudotannins [A].
Another 25ml of extract was mixed with 15ml of gelatin solution in a 100ml measuring flask and complete to volume with water and filtered. To the 20ml filtrate, 25ml of 0.1N iodine and 10ml of 4% NaOH were added. This was mixed and kept in the dark for 15minutes.
The mixture was diluted with 10ml water and acidified with 4% H2SO4 (10ml) then titrated with 0.1N sodium thiosulphate using starch as indicator. The numbers of mls of 0.1N Na2S2O3 used correspond only to pseudotannin content [B]. A blank experiment was carried using distilled water.
Calculation
1ml of N/10 Na2S2O3 ≡ 1ml of N/10 I2 solution ≡ 0.0290g of tannin
% of total = [Blank – ExpA] x 0.029 x 100/5(volume taken)
%pseudotannins= [Blank – ExpB] x 0.029x 100/5ml (volume taken)
% True tannin = [A – B] = g%W/V.
Determination of Saponins
To 50ml of plant extract were placed in a 500ml flask. 300ml of 50% alcohol were added and boiled under reflux for 30minutes and filtered while hot through a filter paper. 2g of charcoal were added to the filtrate, boiled and filtered while hot.
The filtrate was cooled and an equal volume of acetone was added to completely precipitate the saponin. The precipitated saponin was collected by decantation and dissolved in small amount of boiling 95% alcohol and filter while hot. The filtrate was cooled room temperature to separate the saponin in a relatively pure form. The clear supernatant flud was decanted and the saponins suspended in about 20ml of alcohol and filtered. The filter paper was transferred to a dessicators containing anhydrous calcium chloride and left to dry weighed.
Calculation
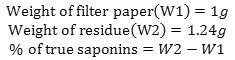
Determination of Cardiac glycosides
8ml of plant extract was transferred to a 100ml volumetric flask and 60ml of H2O and 8ml of 12.5% lead acetate were added, mixed and filtered.50ml of the filtrate was transferred into another 100ml flask and 8ml of 47% Na2HPO4 were added to precipitate excess Pb2+ ion. This was mixed and completed to volume with water. The mixture was filtered twice through same filter paper to remove excess lead
phosphate. 10ml of purified filtrate was transferred into clean Erlyn – Meyer flask and treated with 10ml Baljet reagent. A blank titration was carriedout using 10ml distilled water and 10ml Baljet reagent. This was allowed to stand for one hour for complete colour development. The colour intensity was measured colorimetrically at 495nm.
Calculation
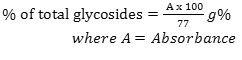
Results and Discussion
The present study carried out on the seed kernel revealed the presence of medicinally active constituents.
Table 1: Qualitative Phytochemical Content of ChrysophyllumalbidumSeed Kernel.
| Phytochemicals | Status |
| Alkaloids | + |
| Flavonoids | + |
| Tannins | + |
| Terpenes | – |
| Cardiac glycosides | + |
| Saponins | + |
| Anthraquinones | – |
| Steroids | – |
Key
Presence of constituent s= +
Absence of constituents = –
Alkaloids, tannins, flavonoids, saponins, and cardiac glycosides were all present in the plant seed. Anthraquinones, terpenes and steroids were absent in the plant.
Table 2: Quantitative phytochemicals content ofChrysophyllumalbidum seed kernel.
| Phytochemicals | Percentage composition (g/100g) |
| Alkaloids | 3.56 |
| Flavonoids | 0.15 |
| Tannins | 2.184 |
| Saponins | 0.24 |
| Cardiac glycosides | 1.88 |
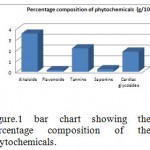 |
Figure 1: bar chart showing the percentage composition of the phytochemicals. |
 |
Figure 2: Chrysophyllum albidum fruit. |
 |
Figure 3: Chrysophyllum albidum Seed Kernel. |
Discussion
The phytochemical screening and quantitative estimation of the crude yields of chemical constituents of the plant part studied were rich in alkaloids, flavonoids, tannins, cardiac glycosides and saponins. They were known to show medicinal activity as well as exhibiting physiological activity.
The presence of alkaloids in seed cotyledon has also been reported by other researchers and this plant has anti-inflammatory effect which helps control all indications of gastritis, esophagitis, enteritis and irritating bowel disorders. Seed cotyledon contains high alkaloids and tannins and this confirm with the reportof [19, 20]. The latter also observed that some of the sapotaceae species including Chrysophyllumdelevoyi are used for treating fibroid when grind, mix with water and potach or alcohol and potach. They are also used in the treatment of gonorrhea and hay fever. The seed cotyledons possess very high levels of alkaloids and tannins and the latter show anti-allergic, anti-inflammatory, anti-microbial and anti-cancer activity.
The seed cotyledon phyto-constituents were dominated by alkaloids and tannins (3.56g/100g and 2.184g/100g) respectively, and that of the cardiac glycoside is 1.88g/100g then followed by those of saponins and flavonoids (0.24g/100g and 0.15g/100g) respectively. The high tannin content of this plant may be responsible for the ethno-medicinal usage. The alkaloids may be toxic chemical element in the seed cotyledon, used as a remedy for fever. This confirms the efficacy seed against vaginal infection. This further explains the therapeutic and medicinal properties of Chrysophyllumalbidum and supported the used of this plant as an external application for skin eruptions diseases. It has been observed that tannins are responsible for anti-diarrheal activity and saponins used as dietary supplements expectorant and anti-inflammatory agent. Evaluation of the potentials of Chrysophyllumalbidum in wound care showed that the cotyledon extract exhibited haemostatic, antimicrobial and wound healing activities. The cotyledon extract mixed with shear butter oil arrested bleeding from fresh wounds by reducing bleeding and clotting time. The haemostatic effects of the extract may be due to increase in the coagulation process with the consequent reduction in clotting time as well as vasoconstriction which are necessary in limiting blood loss from damaged vessels. The phytochemicals may contribute to the wound healing activity by suppressing inflammatory reaction involved by injured tissues.
The value of alkaloids was very high in the seed cotyledon 3.56g/100g. Alkaloids are potent water soluble super antioxidant and free radical scavengers which prevent oxidative cell damage, have stronger anticancer activity and inhibit tumour growth. A study has shown that alkaloids can inhibit the development of fluids that result in diarrhea by targeting the intestinal tracts lower the risk of heart disease. The biological functions of alkaloids include protection against allergies, inflammatory, free radical scavenging, platelet aggregation, microbes, ulcers, hepatoxins, viruses and tumours. This account for the natural anti-oxidants by acting against oxidative stress related disease such infections as; diabetic, cancer, coronary heart diseases. Hence, people that are prone to such infections can feed on Chrysophyllumalbidum fruit as source of natural antioxidants.
Conclusion
The result of this study indicate that the extracts of the seeds of Chrysophyllumalbidum have good potentials as anti-inflammatory, anti-cancer, anti-diarrheal and anti-hemorroidal compound and further provide a rationale for the use of the seed extracts of this plant in traditional medicine.
The plant part studied here has also been as a potential source of useful drugs. Further studies are going on these plants in order to isolate, identify, characterize and elucidate the structure of the bioactive compounds.
Acknowledgement
The authors are grateful to Department of Biochemistry Bayero University Kano Nigeria, for providing necessary chemicals and equipment’s for the completion of the work.
References
- Aliyu, B.S (2006). Common ethnomedicinal plants of the samarids regionOf West Africa their description and phytochemicals.TrumphPublishing Company limited Kano Nigeria. Pp: 180-181.
- Sofowara, A. (1993). Medicinal plants and medicine in Afric john willeySpectrum, Ibadan.Nigeria Pp.2, 81-85.
- Dalziel,J.M. (1937). The useful plant of work tropical Africa.Crown Agent for the colonies. London. Pp: 524.
- Amusa, N.A., Ashaye, O.A and Oladapo, M.O., (2003). Biodeterioration of African star apple (Chrysophyllumalbidum) in storage and the Effect on its food value.Afr. J Biotechnol. 2:56-59.
- Okafor, J.C., (1975). The place of wild (uncultivated) fruits and vegetables in the Nigerian diet. Proceedings, Recommendations and papers of the first National seminar on fruits and vegetables. Ibadan Nigeria, Pp: 153-1154.
- Pearson, D. (1976). Chemical analysis of foods. Churchill livingstone, London. Pp: 181.
- Nwadinigwe, C.A., (1982). Nutritional value and mineral contents ofChrysophyllumalbidumfruit.J.SCI. Food APIC. 1952. 33,283-28G.
- Adisa,S.A., (2000). Vitamin C, protein and mineral content of African starapple (Chrysophyllumalbidum) in preceedings of the 18thAnnual conference of NIST. (eds). Pp: 141-146.
- Adewusi,H.A., (1997). The African star apple Chrysophyllumalbidumindigenous knowledge from Ibadan, south western Nigeria in preceeding of a National workshop on the potentials of theStar apple in Nigeria (eds). Pp: 25-33.
- Burits,M and Bucar, F. (2002). Antioxidant activity of Chrysophyllumalbidum essential oil. Phtother Res 14:323-328. Institute of pharmacognosy.
- Halliwell, B., (1994). Free radicals and antioxidant: a personal view. Nutr., 52: 253-265.
- Parasakthy, N., Boey, C., Goh K.L., (1996). An endoscopic evaluation of recurrent abdominal pain in malaysia. Malaysian journal of child health.8:23-27.s
- Adeyinka A, Liang, H and Tina, G. (2007).Removal of metal ion from wasteWater with Natural waste, School of Engineering and Technology.33:1-8.
- Trease, G.E and Evans, W.C (1989).Treaseand Evans.Pharmacognosy, 13th BelliereTindall. London Pp: 386.
- Sofowara, A. (1982). Medicinal plants and medicine in afric john willey spectrum, Ibadan. Nigeria Pp.2, 101-107.
- Adisa,S.A., (2000). Vitamin C, protein and mineral content of African starapple (Chrysophyllumalbidum) in preceedings of the 18thAnnual conference of NIST. (eds). Pp: 141-146.
- Hassan, M.M; Oyewale, Amupitan A.O. Abdullahi, I.O. Abdullahi, M.S. and Okwonwo, E.M. (2005). Preliminary phytochemical and antibacterial investigation of the root bark of Detariummicrocarpum, J. Chem, Soc. Nigeria 29 (1): 26-29.
- El-Olemy, M.M., F.J. Al-muhtadi and A.A. Afifi, 1994.Experimental Phytochemistry. A laboratory manual. King saud UniversityPress, Saudi Arabia, Pp: 3-19.
- Idowu, O., Iwalewa, E.O., Aderogba, M.A., Akinpelu B.A. and OgundamiA.O. (2006). Antinociceptive, anti-inflammatory And anti-oxidant activities of Eleagnine: An alkaloid isolated From Chrysophyllumalbidum seed cotyledons.Journal of Biological Sciences, vol. 6(6,), Pp: 1029-1034.
- Faleyimu, O.I. and Oluwalana, S.A. (2008).Medicinal value of foresplantseeds.Ogun state, Nigeria. Pp: 276.
(Visited 5,759 times, 1 visits today)

This work is licensed under a Creative Commons Attribution 4.0 International License.





