Manuscript accepted on : 09-01-2020
Published online on: 05-09-2020
Plagiarism Check: Yes
Reviewed by: Ibrahim Hmmam
Second Review by: Heba Mahmoud ![]()
Final Approval by: Dr. Ghulam Md Ashraf ![]()
![]()
Standardization of Mannose Based Positive Selection in indica Rice Variety Swarna
Sai Krishna Repalli*, Chaitanya Kumar Geda and GJN Rao
ICAR-National Rice Research Institute, Cuttack, India-753006
Corresponding Author E-mail : saikrishnarepalli@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2868
ABSTRACT: Successful transgenics require stringent production of large number of successful transgenic events where there is no solution from gene pools of donar varieties. However transgenic technology is a sequential, cumbersome and expensive process. Moreover, it is time consuming, one has to wait for the inheritance of successful transgene into the next generation. Selectable marker genes will play a pivotal role in transient gene confirmation. In the context where the application of herbicide/antibiotic genes as selectable markers is limited; Sugar based selection involving phospho mannose isomerase gene will be helpful in screening of the transformed events. Mannose based selection system is evaluated in indica rice and the optimum selection concentration is standardized. The results, prospects and consequences are discussed.
KEYWORDS: indica Rice; Phosphomannose isomerase; Positive Selection; Sugar; Transgenic
Download this article as:| Copy the following to cite this article: Repalli S. K, Geda C. K, Rao G. J. N. Standardization of Mannose Based Positive Selection in indica Rice Variety Swarna. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Repalli S. K, Geda C. K, Rao G. J. N. Standardization of Mannose Based Positive Selection in indica Rice Variety Swarna. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/31YTSPG |
Introduction
Genetically modified (GM) crops are a field reality and many nations had adopted GM crops. Several agronomical traits targeting various biotic and abiotic stresses were successfully incorporated into various crop species.1These traits provide metabolic advantage to the crop species whose native genepool does not harbor specific target genes. Several genes were transferred across the species.2-3 irrespective of the genetic background. However, success of foreign gene transfer depends on the stringent selective criteria, adopted after gene transformation. Predominantly selection of transformed explants is done with the help of antibiotic selectable markers with their added advantage to make the transformed explants survive in the selective antibiotic medium.4-11 However, these antibiotic selectable marker genes pose environmental threats.12-22 Keeping in view, the biosafety aspects of transgenics, positive selection is adopted using nontoxic substances as selectable agents, such as xylose, galactose, and mannose.23-25
In this study we had utilised mannose/phosphomannose isomerase (PMI) system. In this system, man A gene coding for the enzyme phosphomannose isomerase (pmi) taken from Escherichia coli, is used as selectable marker.26 This system allows selection of transformed explants which have a metabolic advantage to utilize mannose sugar in the medium whereas non transformed cells cannot. The selection strategy is based on the observation that the intracellular hexokinase converts mannose into its orthophosphate by utilizing the energy currency of the cell, thereby resulting in feedback inhibition of the cycle and further severe growth inhibition of the cells27-30. However, the enzyme phosphomannose isomerase (pmi) catalyzes the conversion of accumulated mannose orthophosphate into fructose-6-phosphate which can be metabolized by the transformed cells.31 In this kind of selection, non-transformed cells are deprived of the metabolic carbon source, hence their growth is restricted, whereas the transformed counterparts start growing in the mannose medium. However, in antibiotic selection, non-transformed cells are killed due to the toxic effects of antibiotic. Hence antibiotic selection is called as negative selection, whereas mannose based selection is called as positive selection.
In this study PMI gene was transformed into elite indica rice variety Swarna. Mannose is employed as sugar source for selection. Experimental results regarding optimizing selection concentration of mannose and variations followed during regeneration stage compared with other studies along with the prospects and consequences involved were discussed.
Materials and Methods
Genotype
In this study, an elite indica rice variety Swarna, is used for transformation. It is a popular rice variety and is widely grown in eastern Indian and several other states It is widely adapted and grown in neighboring countries like Myanmar and Bangladesh.32 It has a yield potential of 75 Quintals/hectare. The grains are short bold and the duration of the crop is 150 days.33
Callus Induction and Proliferation
Surface sterilization of the mature dehusked grains of indica rice variety Swarna was carried out as per earlier reports.34 Sterilized kernels were then inoculated into culture tubes containing semisolid Callus Induction (CI) medium. [MS medium supplemented with maltose (30 gl-1), 2, 4-dichlorophenoxy acetic acid (2, 4-D) (2 mgl-1), and solidified with gel-rite (2.6 gl-1)]35 later, cultures were kept in dark and incubated at 24 ± 2ºC for three weeks. Compact embryogenic calli were excised and transferred into semi solid modified MS medium made ready for bombardment. (MS salts and vitamins, Myo Inositol (100 mgl-1), Sorbitol, (20 gl-1), Mannitol (36.4 gl-1), maltose (30 gl-1), L-proline (500 mgl-1), casein hydrolysate (300 mgl-1), 2,4-D (2.0 mgl-1), and Gelrite, (2.6 gl-1) with pH 5.8)
Plasmid Preparation
A single colony of E.coli strain pNOV2819 (Syngenta, USA) carrying the man A gene coding for the enzyme phosphomannose isomerase (pmi) was used for culture and plasmid attraction as per our earlier reports.36
Transformation
Micro-carriers were prepared as per standard protocol using gold, particles (1µ).37 Plasmid DNA with the transformation vector pNOV2819 is loaded onto the micro carriers and bombarded on embryogenic calli at 1100 psi helium pressure using the particle gun PDC-1000/He system (BIORAD) following manufacturer’s instructions.
Optimization of Mannose Concentration Required for Selection
With a view to standardize the concentration of mannose for selection of the transformed calli, an experiment was designed using the seed germination and seedling growth as the critera to fix the ideal concentration using different concentrations starting from 0.025% to 1.0% with mannose alone and with combination of other sugars like glucose, sucrose and maltose (Fig.1)
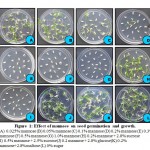 |
Figure 1: Effect of mannose on seed germination and growth. |
Selection
In case of positive selection, after the bombardment, the embryogenic calli were kept at dark for overnight in the same medium. The following day, the transformed calli were sub cultured onto selection medium supplemented with mannose @ 10 g l–. After 15 days, the newly developing calli based on their growth pattern on mannose media were distinguished into actively dividing calli, moderately dividing calli and poorly dividing calli and were sub cultured onto fresh media for at least four cycles (Fig.2) and after keeping for four cycles on selection medium, the number of actively dividing calli were recorded and transferred into regeneration media.
 |
Figure 2: Differential growth pattern of calli grown on mannose supplemented media |
DNA Extraction and PCR assay
Actively growing calli on the selection media were selected and from a half portion of the callus, DNA was extracted following the mini prep method38 while, the second half of the callus was allowed to grow in the medium. Incidence of PMI gene was determined by polymerase chain reaction (PCR) with the help of specific primers to give an amplification product of ~0.514 kb size. The plasmid DNA (pNOV2819) was used as the positive control and non-transformed callus DNA is taken as negative control. The PCR mix contained 1µl of plant DNA (20ng), 0.8µl of 2.5mM dNTPs (Fermentas), 1.0 µl of 10X PCR buffer (10mM Tris, pH 8.4, 50 mM KCl, and 15 mM MgCl2; Sigma), 0.2µl of Taq DNA Polymerase (5U/µl Sigma), 1 µl each of both forward and reverse primers (5 pico moles/µl Sigma) and 5 µl of autoclaved sterile distilled water in a total volume of 10 µl. The amplification was done in a thermal cycler (Eppendorf Vapo protect) under following conditions: an initial denaturation of template DNA at 940C for 3 min followed by 35 cycles of amplification i.e., 1 min denaturation at 940C, 10 min primer annealing at 600C, 2 min primer extension at 720C and 10 min final primer extension at 720C. PCR products were segregated in 1.2 % agarose gel (in 1X TBE electrophoresis buffer) containing 0.5 mg/ml ethidium bromide. Size of the separated PCR products was examined by visualizing under UV light and recorded by gel documentation system (Alpha innotech).
Results
Evaluation of Mannose on Seedling Growth
The results suggest that the growth of seedlings was inhibited from 0.3% until 1.0% concentration of mannose. When mannose was evaluated in combination with sugars, seedling’s growth was not affected till 0.2-0.3% mannose concentration while above that concentration, seedling growth was affected (Fig.1).
Molecular Analysis
Molecular analysis was performed to detect the presence of PMI gene in the transformed calli through PCR amplification. Using specific primers, we had confirmed the presence of the gene of interest as a 514 bp amplification product which was visible in the sample numbers 1, 2, 3, 5 and 6 while no amplification was detected in the remaining samples (Fig.3).
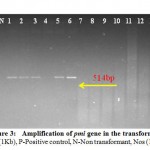 |
Figure 3: Amplification of pmi gene in the transformed plants |
Discussion
In this study, experiments were conducted to study the influence of mannose on the growth of seedlings using various concentrations of mannose starting from 0.2% in combination of other sugars, but seed germination is arrested at 1% mannose concentration and this concentration was employed for the selection. The selection system employed in the study varied from other studies, as most of the researchers have used either sorbitol39 or sucrose40 in addition to mannose for selection, but in doing so, it is difficult to determine the selection concentration of mannose which can restrict the growth of non-transformed cells.
Sucrose in combination with mannose has metabolic advantage where sucrose is readily available in situations where mannose inhibits growth. Hence the combination of both these sugars is utilized in the previous reports41-42, but all the earlier studies focused on precautionary measures to enable transgenic cells to survive on selection medium by addition of metabolisable sugars like sucrose and sorbitol because concentrations of mannose employed in those studies totally depletes the orthophosphate and makes ATP unavailable for the cells to grow, and further growth is retarded. In our study we did not employ any other metabolisable sugars to enhance the growth of transformed cells because this could have drawbacks like production of escapes, but we have utilized low concentrations (1%) of mannose alone per selection and this system worked well as per the growth of callus is concerned (Fig.2) and regeneration and rooting is done on maltose media in accordance with earlier reports. Thus through this study a sugar based positive selection can successfully replace selection using antibiotics which are known to cause environmental problems. The selection system used in this study is optimal for growth of transformed callus and standardized for indica rice cultivar Swarna.
Conclusion
This study holds significance, as it can serve as reference for the standardized protocol while utilizing mannose as selective agent during transformation of indica rice. Specifically, mannose (1%) without any other sugar combination can be effectively utilized during selection and regeneration of transformed calli with phosphomannose isomerase (PMI) system. This positive selection system is advantageous over the negative/antibiotic selection as it does not pose any environmental hazards.
Acknowledgement
The authors are thankful to Director, NRRI for the facilities and encouragement. The first two authors are also thankful to ICAR-NPTC for providing them Senior Research Fellowship.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- James, C. Global status of commercialized biotech/GM crops: (International Service for the Acquisition of Agri-biotech Applications (ISAAA), Ithaca, NY, USA., (2014).
- Pontiroli, A., Simonet, P., Frostegard, A., Vogel, T.M., and Monier, J.M. Fate of transgenic plant DNA in the environment. Environ. Biosafety Res., 6: 15–35 (2007).
- Rizzi, A., Raddadi, N., Sorlini, C., Nordgrd, L., Nielsen, K.M, and Daffonchio, D. The stability and degradation of dietary DNA in the gastrointestinal tract of mammals: implications for horizontal gene transfer and the biosafety of GMOs. Crit Rev Food Sci Nutr.,52(2):142‐161 (2012).
- Chan, M.T., Chang, H.H, Ho, S.L, Tong, W.F, and Yu, S.M. Agrobacterium-mediated production of transgenic rice plants expressing a chimeric alpha-amylase promoter/beta-glucuronidase gene. Plant Mol Biol., 22:491–506 (1993).
- Potrykus, I and Spangenberg, G. Gene transfer to plants. Springer-Verlag, Berlin., (1995).
- Aldemita, R.R, and Hodges, T.K. Agrobacterium tumefaciens-mediated transformation of japonicaand indica rice varieties. Planta., 199: 612– 617 (1996).
- Dong, J.J., Teng, W.M., Wallace, G., and Timothy, C Agrobacterium mediated transformation of javanica rice. Mol Breed., 2:261–276(1996).
- Burkhardt, P.K., Beyer, P., Wünn, J., Kloti, A., Armstrong, G.A., Schledz, M., von Lintig, J. and Potrykus, I. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J., 11: 1071–1078 (1997).
- Cheng, X., Sardana, R., Kaplan, H., and Altosaar, I. Agrobacterium-transformed rice plants expressing synthetic cryIA(b) and cryIA(c) genes are highly toxic to striped stem borer and yellow stem borer. Proc Natl Acad Sci., 95: 2767–272(1998).
- Haldrup, A., Petersen, S.G, and Okkels, F.T. The xylose isomerase gene from Thermoanaerobacterium thermosulfurogenes allows effective selection of transgenic plant cells using D-xylose as the selection agent. Plant Mol Biol., 37:287–296(1998).
- Ye, X., Al-Babili, S., Kloti, A., Zhang, J., Lucca, P., Beyer, P., and Potrykus I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science., 287:303–305 (2000).
- Wang, A. S., et al. A mannose selection system for production of fertile transgenic maize plants from protoplasts. Plant Cell Rep.,19: 654–660 (2000).
- Kuiper, H.A., Kleter, G. A., Noteborn, H.P.J.M and Kok, E.J. Assessment of the food safety issues related to genetically modified foods. Plant J., 27: 503–528 (2001).
- Stewart, C.N., Halfhill, M.D. and Warwick, S.I. Transgene introgression from genetically modified crops to their wild relatives. Nat. Rev. Genet.,4: 806–817(2003).
- Nakamura, Y., Itoh, T., Matsuda, H and Gojobori T. Biased biological functions of horizontally transferred genes in prokaryotic genomes. Nat. Genet., 36: 760–766. (2004).
- Gadaleta, A., Giancaspro, A., Blechl, A., and Blanco, A. Phosphomannose isomerase, pmi, as a selectable marker gene for durum wheat transformation. Journal of Cereal Science., 43: 31-37 (2006).
- Pontiroli Alessandra., Simonet Pascal., Frostegard Asa., Vogel Timothy., and Monier Jean-Michel. Fate of transgenic plant DNA in the environment. Environmental biosafety research., 6: 15-35. (2007).
- Ramessar Koreen., Peremarti Ariadna., Gomez-Galera Sonia., Naqvi Shaista., Moralejo Marian., Munoz-Odina Pilar., Capell Teresa and Christou Paul. Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: A case of the science not supporting the politics. Transgenic research., 16: 261-80 (2007).
- Didelot, X and Maiden, M.C.J. Impact of recombination on bacterial evolution. Trends Microbiol.,18: 315–322 (2010).
- Kwit, C., Moon, H.S., Warwick, S.I and Stewart, C.N. J. Transgene introgression in crop relatives: molecular evidence and mitigation strategies. Trends Biotechnol., 129: 284–293 (2011).
- Nicolia, A., Manzo, A., Veronesi, F and Rosellini, D. An overview of the last 10 years of genetically engineered crop safety research. Crit. Rev. Biotechnol., 34: 77–88 (2014).
- da Silva Dias, J.C. Plant breeding for harmony between modern agriculture production and the environment. Agr. Sci., 6: 30 (2015).
- Haldrup Anna., Noerremark Michael, and Okkels Finn. Plant selection principle based on xylose isomerase. In Vitro Cellular & Developmental Biology – Plant.,37: 114-119 (2001).
- Privalle Laura., Wright Martha., Reed Janet., Hansen Genevieve., Dawson John., Dunder Erik., Chang Yin-Fu., Powell, M and Meghji, Moez. Phosphomannose Isomerase, A Novel Selectable Plant Selection System: Mode of Action and Safety Assessment. Ann. NY Acad. Sci., 964: 129–138 (2002).
- Joersbo, M., Jorgensen, K., and Brunstedt, J. A selection system for transgenic plants based on galactose as selective agent and a UDP-glucose: galactose-1-phosphate uridyltransferase gene as selective gene. Mol Breed., 11: 315–323 (2003).
- Miles, J.S and Guest, J.R Nucleotide sequence and transcriptional start point of the phosphomannose isomerase gene (manA) of Escherichia coli. Gene., 32: 41–48 (1984).
- Ferguson, J.D., Street, B.E, and David, S.B. The carbohydrate nutrition of tomato roots. Annals of Botany., 22: 525–538 (1958).
- Goldsworthy, A and Street, H.E. The carbohydrate nutrition of tomato roots: VIII. The mechanism of the inhibition by D-Mannose of the respiration of excised roots. Ann. Bot.-London., 29: 45–58 (1965).
- Malca, I., Endo, R.M, and Long, M.R. Mechanism of glucose counteraction of inhibition of root elongation by galactose, mannose and glucosamine. Phytopathology., 57: 272–278 (1967)
- Sheu-Hwa, C.S., Lewis, D.H., and Walker, D. A. Stimulation of photosynthetic starch formation by sequestration of cytoplasmic orthophosphate. New Phytologist., 74: 383- 392 (1975)
- Joersbo, M., Petersen, S.G, and Okkels, F.T. Parameters inter-acting with mannose selection employed for the production of transgenic sugar beet. Plant Physiology.,105: 109-116 (1999)
- Baisakh, N., Datta, K., Oliva, N., Ona, I., Rao, G.J.N., Mew, T.W, and Datta, S.K. Rapid development of homozygous transgenic rice using anther culture harboring rice chitinase gene for enhanced sheath blight resistance. Plant Biotechnology., 18: 101-108 (2001)
- Rao, V.R., Reddy, P.S., Murthy, N., Rao, I., Rao, P.S., Rao, C.B., Rao, G.M. Swarna (MTU 7029)–a new stable hybrid with wide adaptation. Oryza., 20:240–242 (1983)
- Vijayachandra, K., Palanichelvam, K., and Veluthambi, K. Rice scutellum induces Agrobacterium tumefaciens vir genes and T-strand generation. Plant Molecular Biology., 29: 125–133 (1995)
- Murashige, T., and Skoog, F. A. revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant physiology., 15: 473-497 (1962)
- Saikrishna, R., Chaitanya, K.G and Rao, G.J.N. Efficacy of different transformation methods in rice (Oryza sativa ). Journal of Experimental Biology and Agricultural Sciences 1: (2S).,106-110 (2013).
- Repalli, S.K., Geda, C.K, and Rao, G.J.N. MMT, a High Promising, Cost Effective Micro-carrier for Gene Delivery. J Microb Biochem Technol.,11: 417(2019).
- Dellaporta, S.L, Wood, J, and Hicks, J.B. A plant DNA mini preparation: Version II. Plant Mol Biol Rep.,14:19-21 (1983).
- Juliana, D., Annika, P., Jurith, M., and Iris, S. The use of the phosphomannose-isomerase/mannose selection system to recover transgenic apple plants. Plant Cell Reports., 25:1149-1156 (2006).
- Lucca, P., Ye, X., and Potrykus I. Effective selection and regeneration of transgenic rice plants with mannose as selective agent. Molecular Breeding.,7:43–49 (2001)
- Joersbo, M., Donaldson, I., Kreiberg, J., Petersen, S.G., Brunstedt, J., and Okkels, F.T Analysis of mannose selection used for transformation of sugar beet. Molecular Breeding., 4: 111-117 (1998).
- Suprasanna, P., Manjunatha, B.R., and Bapat, V.A. Mannose based selection with phosphomannose isomerase (PMI) gene as a positive selectable marker for Rice genetic transformation. Journal of Crop Scienceand , 11 (4): 233-236. (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.





