Manuscript accepted on : 18 November 2017
Published online on: --
Plagiarism Check: Yes
Synthesis, Electrochemical and Antimicrobial Activity of Colloidal Copper Nanoparticles
Basma Al-Johani2, Amna N. Khan2 , Zahra M. Alamshany2, Munazza Gull3
, Zahra M. Alamshany2, Munazza Gull3 , Elham S. Azam2, Samia A. Kosa2
, Elham S. Azam2, Samia A. Kosa2 and M. Tahir Soomro1
and M. Tahir Soomro1
1Center of Excellence in Environmental Studies, King Abdulaziz University, Jeddah, Saudi Arabia.
2Department of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
3Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
Corresponding Author E-mail: amnankhan@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2568
ABSTRACT: The colloidal dispersion of copper nanoparticles (CuNPs), prepared by reducing Cu2+ ions using ascorbic acid, was characterized and used for electrochemical and antimicrobial activity investigations. By depositing CuNPs onto the glassy carbon electrode (GCE) surface the CuNPs/GCE was constructed, which was used to study electrochemical behavior of CuNPs and to carry out direct electrochemical detection of trichloroacetic acid (TCA) and 2-chlorophenol (2-CP) in neutral medium. Excellent electrocatalytic ability of CuNPs, assessed by cyclic voltammetry (CV), for the reduction of TCA and 2-CP was detected. The electrochemical impedance analysis (EIS) of the GCE and CuNPs modified GCE evidenced higher charge transfer activity across the modified electrode surface. The antibacterial activity tests of as-synthesized CuNPs on the selected pathogenic strains of pathogenic strains of Salmonella group B (7.9±0.912), Klebsiella pneumonia (8.33±1.561), Escherichia Coli (15.65±1.612), Enterococcus faecalis (5.4±0.612), Staphylococcus aureus (12.6±1.531) and yeast Candida albicans (11.4.3±1.512), respectively, were performed. The results indicated that the use of CuNPs can be pursued as an alternative strategy (to antibiotics) for averting infections by controlling bacterial adhesion and bacterial bio-film formation against microbial infections.
KEYWORDS: Aqueous Dispersion; Antimicrobial Activity; CuNPs; Electrochemical Detection; Enterococcus Faecalis
Download this article as:| Copy the following to cite this article: Al-Johani B, Khan A. N, Alamshany Z. M, Gull M, Azam E. S, Kosa S. A, Soomro M. T. Synthesis, Electrochemical and Antimicrobial Activity of Colloidal Copper Nanoparticles. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Al-Johani B, Khan A. N, Alamshany Z. M, Gull M, Azam E. S, Kosa S. A, Soomro M. T. Synthesis, Electrochemical and Antimicrobial Activity of Colloidal Copper Nanoparticles. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28320 |
Introduction
There is a great concern among researchers about the air stable and aqueous dispersion synthesis of CuNPs to utilize them for a variety of applications including photochemical catalysis, biosensing, and electrochemical sensing.1-3 Many methods such as chemical reduction, evaporation deposition, thermal decomposition, microwave irradiation, reverse micelles, micro-emulsion, sonochemical reduction, and electrochemical synthesis currently being used to prepare CuNPs.4 Among all these methods the aqueous solution synthesis, achieved by reduction of Cu2+ precursor ions, is believed to be the most simple, cost-effective, and environmentally friendly approach.5 Moreover, the conditions to obtain fine particles with high yield are easy to tune. The drawback of this method includes the readily oxidation of CuNPs in the atmosphere and in the aqueous solvents, which can be avoided by using protective agents.6 Generally, non-toxic protecting agents, which are inexpensive and renewable, showed great promise when they were used for the green synthesis of CuNPs. Furthermore, the green chemicals with combined reducing and protecting capabilities such as ascorbic acid have gained ample attraction in recent studies, and the synthesis of CuNPs with one chemical is a straightforward job.7
The electrochemical activity of CuNPs is best known, and CuNPs based electrode materials are widely used at various levels in electrochemical applications particularly for electrochemical sensing of glucose. For example, Pop et al.8 demonstrated a non-enzymatic detection of glucose using copper-decorated multiwall carbon nanotube composite electrode (Cu/CNT-epoxy). Martin-Yerga et al.9 synthesized copper modified titanium phosphate nanoparticles (CuTiPNPs) as electrocatalyst for glucose detection. Male et al.10 fabricated a composite of copper nanoparticles with single-wall carbon nanotubes (SWCNTs) for electrochemical detection of carbohydrates. Wang et al.11 constructed a Cu/MWCNT-paste electrodes for amperometric detection of carbohydrates and amino acids following their capillary-electrophoresis microchip separation. Bare CuNPs based electrode platform was also reported and shown to facilitate electrochemical reduction of nitrate and nitrite. Davis et al.12 reported the electrochemical detection of nitrate and nitrite at a copper modified electrode, and13 electrochemical detection of nitrate at a copper modified electrode under the influence of ultrasound. However, to the best of our knowledge, no report has been found about the electrochemical detection of TCA and 2-CP using CuNPs modified electrode.
In concern over public health, limiting the amount of microbial contamination in drinking water is one of the crucial environmental problem.14-15 Therefore, development of new materials with improved antimicrobial properties is essential for monitoring and controlling microbial activities, food packaging purposes, and producing sterile surfaces.16-18 In past few years, metal nanoparticles such as Ag, Cu etc., showed great promise when used as antimicrobial agents against microorganisms.19-22 Metal nanoparticles are the most effective antimicrobial agents used for antimicrobial activities because of their small size, high surface to volume ratio, and ability to interreact closely with microorganisms. Since CuNPs have great significance in diverse fields, therefore, their use, especially as antimicrobial agents, have been the focus of current research,23-24 and searching for the air stable synthesis of an aqueous dispersion of CuNPs is of considerable interest. The gram-positive bacterium Enterococcus faecalis can cause a variety of infections and the most common was urinary tract infection.25-26 The many isolates of Enterococcus faecalis were found drug-resistant and therefore, the infections caused by Enterococcus faecalis are extremely difficult to treat. On the other hand, the antibiotic-resistant strains of Staphylococcus aureus such as methicillin-resistant Staphylococcus aureus (MRSA), which is an emerging threat to human health, can cause a variety of problems including skin and soft tissue infection.
27 However, despite much research and development, there is no approved vaccine is available to prevent invasive Staphylococcus aureus. disease. To date, the antibacterial activity of CuNPs against Enterococcus faecalis, Staphylococcus aureus and other bacterial strains has been reported only in a few studies, and the application of CuNPs in medicine as an antibacterial agent and in other various fields such as medical instrument and devices still required a great deal of research.
In this study, highly stable dispersion of CuNPs was synthesized by aqueous solution reduction of Cu2+ ions using ascorbic acid as reducing and protecting agent. The characterization studies were carried out using UV-Vis, FTIR, SEM and EIS techniques. For electrochemical detection of TCA, and 2-CP, the bare CuNPs were deposited onto the surface of GCE to prepare CuNPs/GCE, and the sensing applicability of the prepared electrode was tested. The described bare CuNPs modified electrode has advantages over previous copper electrodes and extended the analytical application of CuNPs to a wide variety of analytes. Then, the antibacterial activity test against various infectious pathogenic microorganisms such as Salmonella group B (7.9±0.912), Klebsiella pneumonia (8.33±1.561), Escherichia Coli (15.65±1.612), Enterococcus faecalis (5.4±0.612), Staphylococcus aureus (12.6±1.531) and yeast Candida albicans (11.4.3±1.512) was performed, and the results obtained were promising.
Experimental Details
Materials
Copper(II) sulfate pentahydrate (CuSO4·5H2O), ʟ-ascorbic acid, sodium sulfate anhydrous (Na2SO4), sodium chloride (NaCl), trichloroacetic acid (TCA) and 2-chlorophenol (2-CP) purchased from Sigma-Aldrich were of high-grade reagents and used as received. All Solutions were prepared with double distilled water. TCA and 2-CP stock solutions were diluted using 0.1 M sodium sulfate electrolyte to prepare working solutions of desired concentrations. The colloidal CuNPs were prepared by reducing Cu(II) precursor salt using ʟ-ascorbic acid as reducing and protecting agent. In a typical synthesis, 1 ml of 0.3 M Cu2+ was stirred with 1.2 ml of 0.5 M ascorbic acid and 2.8 mL double distilled water at 80 oC. The solution was kept uniform under stirring till the color changed to dark. The resulting CuNPs aqueous dispersion, which was used for assessing electrochemical and antimicrobial activity, found stable under ambient conditions for 3 months.
Characterizations and Electrochemical Measurements
The CuNPs dispersion was characterized by optical absorption using UV-Vis Double Beam PC Scanning Spectrophotometer (Model UVD 2960, Lamomed, Inc.). The organic functional group analysis of ʟ-ascorbic acid and CuNPs was performed with a Perkin Elmer Fourier Transform Infrared (FTIR) spectrometer 100 series (Beaconsfield, Bucks, UK) in the range of 600-4000 cm-1. The visualization of CuNPs was carried out with high resolution scanning electron microscope (SEM) FEI MagellenTM 400. To prepare CuNPs deposited silicon wafer, colloidal dispersion of CuNPs was dropped coated on a silicon wafer and air dried.
Electrochemical performances of CuNPs were evaluated using a multi-channel potentiostat/galvanostat (VSP, Bio-logic Science Instrument, France). For electrochemical data acquisition, analysis, and fitting a modular EC-Lab software shipped with VSP was used. An electrochemical cell consisting a glassy carbon working electrode (GCE), an Ag/AgCl reference electrode, and a Pt wire auxiliary electrode was used. The supporting electrolyte was a 0.1 M sodium sulfate solution which was saturated with nitrogen before each experiment. Cyclic voltammograms (CVs) were recorded between two set points at a scan rate of 100 mV/s. The electrochemical impedance spectroscopy (EIS) measurements, where the charge transfer resistance is recorded by scanning the frequency, were conducted in the frequency range of 1 Hz -100 kHz with an AC amplitude of 10 mV.
The GCE was polished with alumina slurry (0.05 µm), followed by ultrasonic cleaning in ethanol and water for 3 min and thoroughly washed with double distilled water. To prepare CuNPs modified GCE the CuNPs dispersion was loaded onto the GCE surface as follows: 0.2 ml of CuNPs colloidal solution was diluted with double distilled water to 1 ml and sonicated for 15 min. Then, 10 µL of the diluted dispersion was dropped on a GCE surface, and allowed to evaporate solvent at 40 oC to prepare CuNPs/GCE.
Consequently, the electrochemical measurements carried out and TCA and 2-CP were separately detected by CV and EIS. A 10 mL of the 0.1 M sodium sulfate containing 0.1 mM TCA (or 0.1 mM 2-CP) was purged with N2 for 25 min before the measurements. The CV responses of TCA on GCE and CuNPs/GCE was recorded in the potential range of -0.2 V to -1.5 V, whereas CVs for 2-CP were scanned within the potential range of +1.5 V and -1.5 V. All other conditions were same as described above. CV behavior of CuNPs was obtained in 0.1 M sodium sulfate. For EIS measurements, reduction potentials of -1.2 V and -1.28 V for TCA and 2-CP, respectively, were extracted from CV results. The other experimental conditions for EIS were frequency range of 1 Hz to 100 kHz and an AC amplitude of 10 mV.
Antibacterial and Antifungal Activity
The antibacterial and antifungal activity of colloidal CuNPs was assessed by Zone of Inhibition (ZOI) technique. The standard and clinical strains of pathogenic bacteria Salmonella group B, Klebsiella pneumonia (MTCC-4030), Escherichia Coli (MTCC-1677), Enterococcus faecalis (ATCC-29212), Staphylococcus aureus (MTCC-3610) and yeast Candida albicans (MTCC-3017) were used. Clinical samples were obtained from patients with infection from King Abdulaziz University Hospital Jeddah, Saudi Arabia. Nutrient agar slants that contained peptone (5.0 g), yeast extract (2.0 g), meat extract (1.0 g), NaCl (5.0 g), agar (15 g), pH (7.0), and distilled water (1 liter) were used to culture bacterial strains. The final inoculum of all studied organisms was 104 CFU mL−1 (colony forming units per mL). Inhibition zone diameters (mm) were read after 18 h (for bacteria) or 24 h (for yeasts) of incubation at 35 oC. The standard antimicrobial compounds used were ciprofloxacin and kanamycin. The stock fungal culture Candida albicans (MTCC-3017) were maintained on MGYP slants containing malt extract (3.0 g), peptone (10.0 g), and dextrose (10.0 g) per liter of distilled water. The test bacterial and fungal suspensions (50 µL) containing 104 cells mL−1 were spread on MGYP and NA plates, respectively. The experimental details described earlier were follows to perform antimicrobial activity tests.24 100 µL of CuNPs sample, prepared freshly, was added into the wells. The initial incubation time for samples was 15 min at 4 oC (to allow diffusion) and later 24 or 48 h at 37 °C for the bacterial and fungal cultures, respectively. After the incubation period, the zone of inhibition was observed around the well and test results that scored were positive.
The statistical significance of the antibacterial and antifungal tests was determined. All experiments were conducted in triplicate and the statistical test that generate mean values was used. The resulting data is presented in tabular form. For statistical analysis, OriginLab Origin Pro (version 9.0) was used.
Results and Discussion
UV-Vis, FTIR, and SEM Characterization of CuNPs
For the formation of CuNPs, the UV-Vis is an important technique. It is known that the peak position, shape and the particle size are the characteristic features that influence the UV-Vis spectrum of nanoparticles.25-26 The UV-Vis absorption spectrum of CuNPs is shown in Fig. 1a, which was measured in the 300-700 nm range and exhibited two absorption peaks at 320 and 430 nm, respectively. The plasmon absorption peak for CuNPs is expected to appear at around 575 nm, however, when the particles size small enough (≤ 4 nm) the plasmon peak becomes broader and shows featureless appearance at the higher energy side of the UV-Vis spectrum.2-3 Thus, a broadened peak at 430 nm in Fig. 1a, attributed to CuNPs, was due to the formation of very small and separated nanoparticles, as reported elsewhere.1 The result clearly explained that the ʟ-ascorbic acid could serve the purpose of both reducing and protecting agent, and effectively produced smaller CuNPs with long-term air stability, approximately 3 months, and better size disturbing. The absorption peak, indicative of oxidation products of ʟ-ascorbic acid, was appeared at 320 nm.
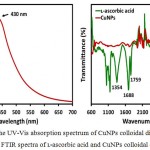 |
Figure 1a: The UV-Vis absorption spectrum of CuNPs colloidal dispersion and (b) FTIR spectra of ʟ-ascorbic acid and CuNPs colloidal dispersion.
|
FTIR spectroscopic measurements were carried out in the range of 600-4000 cm-1 to identify ʟ-ascorbic acid, which was used for reduction of Cu2+ ions and protecting of the CuNPs. Fig. 1b shows the comparison of the FTIR spectra of 0.12 M ʟ-ascorbic acid, which was used to reduce 0.06 M Cu2+ ions in aqueous solution, and the synthesized CuNPs. The FTIR spectrum of the ʟ-ascorbic acid displayed three characteristics peaks at 1354, 1688, and 1759 cm-1, respectively. The broad peak at 3044 cm-1 was due to the O-H stretch of ʟ-ascorbic acid. The stretching vibration peaks at 1354 cm-1 and 1688 cm-1 were assigned to the enol hydroxyl group and C=C, respectively, whereas ester carbonyl group of ʟ-ascorbic acid was responsible for a peak at 1759 cm-1. 1 When the reaction was completed and CuNPs were formed, the peaks for enol hydroxyl group and C=C were almost disappeared, however, one insignificant peak at 1726 cm-1 indicating that oxidized ester carbonyl groups were involved for the adsorption of ʟ-ascorbic acid on the surface of CuNPs. The other evidence for the successful protecting of CuNPs in aqueous solution by ʟ-ascorbic acid was the complete disappearance of O-H stretch peak in the FTIR spectrum of CuNPs.
The scanning electron microscopy (SEM) was used to study the morphology and particle dispersion in CuNPs thin film deposited on a silicon wafer. The typical SEM image of as-prepared CuNPs thin film is shown in Fig. 2. The image showed that CuNPs were spherical in shape and uniformly dispersed on the silicon wafer surface. A uniform dispersion of CuNPs resulted in a homogeneous thin film with improved electrical and mechanical properties,30-31 which was confirmed by CV and EIS measurements.
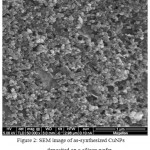 |
Figure 2: SEM image of as-synthesized CuNPs deposited on a silicon wafer.
|
Electrochemical Behavior of CuNPs
The electrochemical behavior of bare CuNPs deposited on the surface of GCE was studied. An example of typical CV, recorded in the potential range of +1.2 V and -1.2 V in 0.1 M sodium sulfate at a scan rate of 100 mV/s, is depicted in Fig. 3a. There is one oxidation peak (-0.035 V) in the anodic direction, which is in accordance with the previous studies and assigned to the formation of Cu2O 32-33 and the peaks associated with the oxidation of Cu2O and CuO were absent. Moreover, no reduction peak in the cathodic direction was appeared, which suggests that the oxidation and reduction of Cu2O or CuO do not take place in sodium sulfate electrolyte. When CuNPs were deposited on the surface of GCE and layered, some of the CuNPs from the upper layer were oxidized and formed an oxide layer of Cu2O and CuO on the surface.34 These oxides are also electrochemically active and reported to produce electrochemical oxidation signals at or near the same electrode potential in an acidic medium where Cu oxidized.35 However, there are conflicting views regarding the order of oxidation of oxides. Some authors have shown that the oxidation of CuNPs takes place first, followed by the oxidation of CuO and Cu2O. While others claimed that Cu2O is oxidized after the oxidation of CuNPs, followed by the oxidation of CuO.34,36 In our case situation is more favorable as we studied oxidation of CuNPs in neutral electrolyte medium, and since to the best of our knowledge there is no previous report about the oxidation of Cu2O and CuO in the neutral electrolyte solution, therefore, this peak is safely related with the oxidation of CuNPs into Cu2O.
Electrochemical Detection of TCA and 2-CP
The sensing ability of the CuNPs modified GCE was investigated using CV and EIS. The analyte chosen were TCA and 2-CP, and their CV behavior on GCE and CuNPs/GCE are represented in Fig. 3a and 3b, respectively. The CVs for reduction of 0.1 mM TCA were recorded in 0.1 M sodium sulfate in the potential range of -0.2 V to -1.5 V at a scan rate of 100 mV/s. With GCE there was no reduction peak for TCA, which indicates that GCE has no ability to electrocatalyze reduction of TCA. However, when CuNPs modified GCE was used to observe reduction of TCA a clear and sharp reduction peak at -1.2 V was appeared, which confirms that the higher catalytic activity of CuNPs. The electrochemical response of 0.1 mM 2-CP was investigated within the potential range of +1.5 V and -1.5 V under the same experimental conditions as for TCA. For 2-CP on GCE, besides one noticeable oxidation peak, there were no clear reduction peaks. When GCE was replaced with CuNPs/GCE, a new reduction peak at -1.28 V for the reduction of 2-CP or oxidized products of 2-CP was observed. This means that a CuNPs modified GCE is also electrocatalyzed the reduction of 2-CP or oxidized products of 2-CP. The improved and better electrochemical performances of CuNPs modified GCE then GCE were attributed to the high surface-to-volume ratio, excellent catalytic activity, and evenly distribution of CuNPs on the surface of GCE. Thus, using CuNPs/GCE electrochemical sensors for simple, fast, and sensitive detection of TCA and 2-CP can be developed in the future.
 |
Figure 3: The CV curves (a & b) and EIS Nyquist plots (c & d) of GCE and CuNPs/GCE for (a) 0.1 mM TCA and (b) 0.1 mM 2CP in 0.1 M sodium sulfate.
|
Electrochemical Impedance Sensing of TCA and 2-CP
The utility of the EIS technique is for the development of the electrochemical sensors and the charge transfer resistance of the modified electrode is a crucial factor to evaluate from the EIS Nyquist plot.37-38 In EIS Nyquist plot, at a higher frequency region, a small semicircle caused by the charge transfer across the modified electrode surface is observed, whereas at the lower frequency region a straight line due to the diffusion of the analyte towards the electrode surface is appeared. The EIS Nyquist plot then fitted and analyzed with an equivalent circuit model, and the important parameters such as electrolyte resistance Rs charge transfer resistance Rct, double layer capacitance Cdl, and Warburg impedance Zw are extracted.39-40
Thus, to further support CV results, EIS measurements of the GCE and CuNPs/GCE were carried out. To see a reduction of TCA, an applied bias of -1.2 V with an AC voltage of 10 mV in the frequency range of 1 Hz-100 kHz was applied. The typical EIS Nyquist plots for the reduction of 0.1 mM TCA at GCE and CuNPs/GCE are shown in Fig. 3c. The CuNPs/GCE revealed smaller semicircle then GCE in the higher frequency region, which means that more molecules of TCA were reached at CuNPs/GCE surface, subsequently reduced, and generated lower Rct. The reduction of 2-CP at GCE and CuNPs/GCE in the form of Nyquist plot conducted at a fixed potential of -1.28 V under the same experimental conditions as for TCA is presented in Fig. 3d. Similarly, in comparison to GCE a small well-defined semicircle at higher frequency region for CuNPs/GCE is obtained, indicating small interface impedance, higher charge transfer ability of the modified electrode, and higher reduction current of 2-CP. These results successfully demonstrated that a lower charge transfer resistance and a higher reduction current for TCA and for also 2-CP was achieved by construction of CuNPs/GCE. The high sensitivity of the CuNPs/GCE toward detection of TCA and 2-CP could be due to the high surface-to-volume ratio of CuNPs and reduced agglomeration of CuNPs in the deposited thin film.
Antibacterial and Antifungal Activity
The antibacterial and antifungal qualities of copper metal are well-reported. However, recently, antimicrobial tests for CuNPs were shown to prevent bacterial adhesion and biofilm formation better than the copper metal itself.41 Thus, antibacterial and antifungal activity tests for as-synthesized CuNPs were carried out and the results are presented in Table 1. The tested bacterial and fungal strains Salmonella group B (7.9±0.912), Klebsiella pneumonia (8.33±1.561), Escherichia Coli (15.65±1.612), Enterococcus faecalis (5.4±0.612), Staphylococcus aureus (12.6±1.531), and yeast Candida albicans (11.4.3±1.512), were markedly affected by the antimicrobial activity of CuNPs. Among all bacterial strains, the Enterococcus faecalis was adversely affected by the toxicity CuNPs and showed higher sensitivity as observed between the inhibition zone observed in disk diffusion and MIC determined based on liquid cultures with the several strains (r2 = 0.75). 42 The effectiveness of the CuNPs as antifungal agents was also probed and the selected fungal stain Candida albicans was found to appear sensitive towards as-synthesized CuNPs. This was realized from the differences in the cell wall of each strain, and the cell wall of gram-positive strain was wider than the cell wall of gram-negative strain.43
Table 1: Comparison of antimicrobial potential of CuNPs on infectious pathogenic microorganisms with standard antibiotics.
| Name of tested samples | Variety of Microorganism | Inhibition zone in diameter (mm) | CuNPs MIC* (µg/mL) |
| Kanamycin | Standard Antibiotic | 5.0 | 0.25 |
| Ciprofloxacin | Standard Antibiotic | 3.7 | 0.25 |
| Salmonella group B | Gram(-) | 7.9 | 0.75 |
| Klebsiella pneumoniae | Gram(-) | 8.33 | 1.012 |
| Escherichia. Coli | Gram(-) | 15.65 | 2.31 |
| Enterococcus faecalis | Gram(-) | 5.4 | 0.85 |
| Staphylococcus aureus | Gram(+) | 12.6 | 2.12 |
| Candida albicans | yeast | 11.4 | 3.15 |
*MIC – Minimal Inhibitory Concentration (µg/mL)
The mechanism of toxicity of CuNPs on various strains is discussed previously. 23-24 based on this, it is inferred here that CuNPs could damage Enterococcus faecalis DNA followed by a reduction of bacterial respiration. Moreover, by altering the conformation and electron transference, CuNPs were appeared to inhibit certain cytochromes in the membrane of Enterococcus faecalis and this could also be the reason for higher sensitivity of Enterococcus faecalis towards CuNPs. The higher antimicrobial activity of silver nanoparticles (AgNPs) against clinical bacterial and fungal pathogenic isolates was reported, and use of AgNPs for the reduction of bacterial adhesion and prevention of biofilm formation was proposed.44 However, in some other studies high antibacterial activity of CuNPs against clinical strain MRSA, even better than AgNPs, and against antibacterial drug ciprofloxacin and kanamycin was demonstrated.44-45 For example, Yoon et al,46 compared antibacterial effects of AgNPs and CuNPs using single representative strains of Escherichia Coli and Bacillus subtilis and found an excellent antibacterial activity for CuNPs than AgNPs. In the present study, the higher antibacterial and antifungal activity of as-synthesized CuNPs against Enterococcus faecalis and Candida albicans were illustrated successfully. However, more detail study is needed to fully understand the bactericidal mechanism of action of CuNPs on various strains. For now, the as-synthesized colloidal dispersion of CuNPs is expected to use as an alternate of AgNPs to control biofilm formation and avoid bacterial adhesion.
Conclusion
The colloidal dispersion of CuNPs was successfully synthesized using a straightforward chemical reduction method and was characterized using standard techniques. The UV-Vis results revealed the formation of small size CuNPs, whereas the successful protecting of CuNPs with ascorbic acid was verified by FTIR analysis. The SEM image showed the uniform dispersion of CuNPs in a thin film formed on a silicon wafer. The electrocatalytic ability of as-synthesized CuNPs was elucidated by using CV and EIS techniques and was utilized for electrochemical sensing of TCA and 2-CP. Antimicrobial tests showed that CuNPs were active against various gram-positive and gram-negative bacteria, and Candida species. The antimicrobial results also suggested the future use of CuNPs in reducing bacterial or fungal adhesion and biofilm formation at low cost. Therefore, this as-synthesized CuNPs has great potential to be used for an environmental and biological application such as in environmental monitoring, for water purification, and as an electrochemical sensing or antibacterial packaging material.
Acknowledgements
The Authors acknowledge support from the Center of Excellence in Environmental Studies (CEES), Department of Chemistry King Abdulaziz University, and the Ministry of Higher Education (MoHE).
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding Sources
This work is funded by Deanship of Scientific Research, King Abdulaziz University, Jeddah, Saudi Arabia.
References
- Xiong Y, Wang Q, Xue X.W. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011;13: 900-904.
CrossRef - Pourbeyram S, Mohammadi. Synthesis and characterization of highly stable and water dispersible hydrogel–copper nanocomposite. J. Non-Cryst. Solids. 2014;402:58-63.
CrossRef - Khan A, Rashid R, Younas R, Chong A. chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016;6:21-26.
CrossRef - Khodashenas H, Ghorbani R. Synthesis of copper nanoparticles: an overview of the various methods, Korean J. Chem. Eng. 2014;31:1105-1109.
CrossRef - Deng, Jin Y, Cheng Y, Qi T, Xiao F. Copper nanoparticles: aqueous phase synthesis and conductive films fabrication at low sintering temperature. ACS appl. Mater. Interfaces. 2013;5:3839-3846.
CrossRef - Liu M, Yasunami T, Kuruda K, Okido M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method, Trans. Nonferrous Met. Soc. China. 2012;22:2198-2203.
CrossRef - Umer S, Naveed N, Ramzan M, Rafique S, Imran M. A green method for the synthesis of Copper Nanoparticles using L-ascorbic acid. Matéria (Rio de Janeiro). 2014;19:197-203.
CrossRef - Pop F,Manea C, Orha S, Motoc E, Ilinoiu N, Vaszilcsin J. Schoonman, Copper-decorated carbon nanotubes-based composite electrodes for nonenzymatic detection of glucose, Nanoscale res. Let. 2012;7:266.
- Martín-Yerga J, Carrasco-Rodríguez J.L, Fierro G.F Alonso J.G, Costa-García A. Copper-modified titanium phosphate nanoparticles as electrocatalyst for glucose detection. Electrochim. Acta. 2017;229:102-111.
CrossRef - Male B.S, Hrapovic Y,Liu D, Wang J.H, Luong T. Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Anal. Chim. Acta. 2004;516:35-41.
CrossRef - Valentini V, Biagiotti C, Lete G, Palleschi J.W. The electrochemical detection of ammonia in drinking water based on multi-walled carbon nanotube/copper nanoparticle composite paste electrodes. Sens. Actuators B: Chem. 2007;128:326-333.
CrossRef - Davis M, Moorcroft J, Wilkins S.J, Compton R.G, Cardosi M.F Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst. 2000;125:737-742.
CrossRef - Davis M, Moorcroft J, Wilkins S.J, Compton R.G, Cardosi M.F. Electrochemical detection of nitrate at a copper modified electrode under the influence of ultrasound. Electroanal. 2000;12:1363-1367.
CrossRef - Ashbolt J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. health rep. 2015; 2:95-106.
CrossRef - Ashbolt J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicol. 2004;198:229-238.
CrossRef - Beyth, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: nano-antimicrobial materials. Evid. based complement. Alternat. Med. 2015;2015.
- Asthuri A.N,Reddy P.M, Roopa D.K, Zamare. Application of Green Synthesized Iron Nanoparticles for Enhanced Antimicrobial Activity of Selected Traditional and Commonly Exploited Drug Amoxicillin Against Streptococcus mutans. Biosci. Biotech. Res. Asia. 2017;14:1135-1141.
CrossRef - Alkammash M. Synthesis of Silver Nanoparticles from Artemisia Sieberi and Calotropis Procera Medical Plant Extracts and Their Characterization using SEM Analysis. Biosci. Biotech. Res. Asia 2017; 14:521-526.
CrossRef - Chalandar E, Ghorbani H.R, Attar H, Alavi S.A. Antifungal Effect of Copper and Copper Oxide Nanoparticles Against Penicillium on Orange Fruit. Biosci. Biotechnol. Res. Asia. 2017;14:279-284.
CrossRef - Kumar A, Palanichamy V, Roopan S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity, Spectrochim. Acta A: Mol. Biomol. Spect. 2014;127:168-171.
CrossRef - Iravani, Korbekandi H, Mirmohammadi S.V, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharma. Sci. 2014;9:385-406.
- Din I, Rehan R. Synthesis, Characterization, and Applications of Copper Nanoparticles. Anal. Lett. 2017;50:50-62.
CrossRef - Tomasz, Szczepanowicz K, Stefańska J, Socha R.P, Warszyński P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles, Colloids Surf. B: Biointerf. 2015;128:17-22.
CrossRef - Ramyadevi K, Jeyasubramanian A, Marikani G, Rajakumar A,Rahuman A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Let. 2012;71:114-116.
CrossRef - Huycke M, Sahm D.F, Gilmore M.S. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future, Emer. Infect. Dis. 1998;4:239.
CrossRef - van Harten M.R,Willems J.L, Martin N.I,Hendrickx A.P.A. Multidrug-Resistant Enterococcal Infections: New Compounds, Novel Antimicrobial Therapies? Trends Microbiol. 2017;6:467-479.
CrossRef - Zetola, Francis J.S, Nuermberger E.L , BishaiW.R. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet infect. Dis. 2005;5:275-286.
CrossRef - Khanna K.S,Gaikwad P.V, Adhyapak N, Singh R.M. Synthesis and characterization of copper nanoparticles. Mater. Lett. 2007;61:4711-4714.
CrossRef - Dang M.D,Le T.T.T, Fribourg-Blanc E, Dang M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2011;2:015009.
CrossRef - Jain A, Jain V.D. Copper nanoparticles catalyzed oxidation of threonine by peroxomonosulfate, J. Saudi Chem. Soc. 2017;21:803-810.
CrossRef - Amin M.T, Soomro N, Memon A.R, Solangi T.Q,Behzad A.R. Disposable screen printed graphite electrode for the direct electrochemical determination of ibuprofen in surface water. Environ. Nanotech. Monit. Manage. 2014;1:8-13.
CrossRef - Zhang J,Yin F, Min L, Jia D,Zhang Q,Zhang J.X. Cyclic voltammetry analysis of copper electrode performance in Na2WO4 solution and optical property of electrochemical synthesized CuWO4 nanoparticles. J. Alloys Compd. 2017;690:221-227.
CrossRef - Valov W,Lu D. Nanoscale electrochemistry using dielectric thin films as solid electrolytes. Nanoscale. 2016;8:13828-13837.
CrossRef - Jayalakshmi K.B. Cyclic voltammetric behavior of copper powder immobilized on paraffin impregnated graphite electrode in dilute alkali solution. Int. J. Electrochem. Sci. 2008;3:1277-1287.
- Darchen R, Drissi-Daoudi A.I. Electrochemical investigations of copper etching by Cu (NH3) 4Cl2 in ammoniacal solutions.J. appl. Electrochem. 1997;27:448-454.
CrossRef - Zhang B, Hua Y.X. Electrochemical synthesis of copper nanoparticles using cuprous oxide as a precursor in choline chloride–urea deep eutectic solvent: nucleation and growth mechanism. Phys. Chem. Chem. Phys. 2014;16:27088-27095.
CrossRef - Al-Qasmi M.T, Soomro I.M.I, Ismail E.Y, Danish A.A, Al-Ghamdi. An enhanced electrocatalytic oxidation and determination of 2, 4-dichlorophenol on multilayer deposited functionalized multi-walled carbon nanotube/Nafion composite film electrode. Arabian J. Chem. 2015. https://doi.org/10.1016/j.arabjc.2015.08.032
CrossRef - Bashami M, Hameed A, Aslam M.I.I, smail M.I, Soomro M.T. The suitability of ZnO film-coated glassy carbon electrode for the sensitive detection of 4-nitrophenol in aqueous medium. Anal. Method. 2015;7: 1794-1801.
CrossRef - Al-Zahrani M.T, Soomro R.M, Bashami A.U, Rehman E, Danish I.M,Ismail I.M, Hameed A. A. Fabrication and performance of magnetite (Fe3O4) modified carbon paste electrode for the electrochemical detection of chlorite ions in aqueous medium. J. Environ. Chem. Eng. 2016;4:4330-4341.
CrossRef - Al-Qasmi M.T, Soomro M, Aslam A.U, Rehman S, Ali E.Y, Danish I.M, Ismail I,Hameed A. The efficacy of the ZnO: α-Fe2O3 composites modified carbon paste electrode for the sensitive electrochemical detection of loperamide: A detailed investigation. J. Electroanal. Chem. 2016;783:112-124.
CrossRef - Dusane H, Rajput J.K, Kumar A.R, Nancharaiah Y.V, Venugopalan V.P, Zinjarde S.S. Disruption of fungal and bacterial biofilms by lauroyl glucose. Lett. Appl. Microbiol. 2008;47:374-379.
CrossRef - Ruparelia P, Chatterjee A.K,Duttagupta S.P,Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles, Acta biomater. 2008;4:707-716.
CrossRef - Thiel L, Pakstis S, Buzby M, Raffi C, Ni D.J, Pochan S,Shah I. Antibacterial properties of silver‐doped titania. Small. 2007;3:799-803.
CrossRef - Szczepanowicz J, Stefanska R,Socha P, Warszynski P. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity, Physicochem. Probl. Miner. Process. 2010;45:85-98.
- Antoszczak E, Maj J, Stefańska J, Wietrzyk J, Janczak B, Brzezinski A, Synthesis H. antiproliferative and antibacterial activity of new amides of salinomycin, Bioorg. Med. Chem. Let. 2014;24:1724-1729.
CrossRef - Ki Y, Yoon J, Byeon H, Park J.H, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007;373:572-575.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





