Manuscript accepted on : 20-04-2023
Published online on: 28-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Sharanabasava V. Ganachari
Second Review by: Dr. Ali Mohamed Elshafei
Final Approval by: Dr. Eugene A. Silow
Bacterial Cellulose: An Ecological Alternative as a Biotextile
Rekha Mehrotra , Samiksha Sharma1
, Samiksha Sharma1 , Nidhi Shree1
, Nidhi Shree1 and Kohinoor Kaur1*
and Kohinoor Kaur1*
Department of Microbiology, Shaheed Rajguru College of Applied Sciences for Women, Delhi University, Vasundhara Enclave, Delhi – 110096, India
Corresponding Author E-mail: Kohinoor.kaur@rajguru.du.ac.in
DOI : http://dx.doi.org/10.13005/bbra/3101
ABSTRACT: Bacterial cellulose has come forth as a novel nano-material with an extensive range of distinct properties, making it an excellent industrial alternative to conventional plant cellulose, as the world moves toward a sustainable and cleaner phase. Bacterial cellulose is a biomaterial that breaks down naturally in the environment and is produced by natural mechanism in bacterial cells. It has been considered as a substitute to traditional biomaterials in numerous sectors, namely, textile, pharmaceutical, food industry, biotechnology, for its features enabling to achieve sustainable development goals. The present focus is on looking at developing an inexpensive substrate for the synthesis of bacterial cellulose from industrial waste as its commercialization is restricted due to social, economic, and environmental considerations. Upcoming research in biotechnological area of biotextiles and biocomposites aims to integrate basic knowledge of textiles with biological sciences thereby facilitating production of goods which are commercially more viable and also less harmful to the environment. The review discusses the data regarding the use of bacterial cellulose and its production over the years, notably in the textile sector, with an emphasis on advancement of research to enable its extensive production and in various other areas like cosmetology, food industry, biomedical and paper industry. In addition, potential benefits of bacterial cellulose development addressing many of the global sustainable development goals along with suggestions for its scale-up have also been discussed.
KEYWORDS: Bacterial Cellulose; Biomaterial; Bio-textile; Clean Biotechnology; Nano-material; Sustainability
Download this article as:| Copy the following to cite this article: Mehrotra R, Sharma S, Shree N, Kaur K. Bacterial Cellulose: An Ecological Alternative as a Biotextile. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Mehrotra R, Sharma S, Shree N, Kaur K. Bacterial Cellulose: An Ecological Alternative as a Biotextile. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3CPX7L3 |
Introduction
Biotechnology products have already made use of several polysaccharides derived from microorganisms that have novel and intriguing biological and physical properties. Cellulose obtained from microorganisms is a potential microbial polysaccharide1. Bacterial cellulose (BC) is a naturally occurring bio-material, synthesised by certain bacterial species, have higher-grade characteristics, and can be modified in the desired manner. Owing to the wide range of properties it offers, BC has become a choice of application in many fields including fashion, engineering, medicine, pharmacy, food, chemistry and environment2. When it comes to fashion products, clothing, accessories, footwear, and other items that have a short life cycle, the number of textiles created and disposed globally is pretty large. The textiles constructed clothes and accessories, including non-woven fabrics, have been interwoven with warp and weft or made from laminated and malleable surfaces using mechanical, chemical, or thermal processes for thousands of years3.
The biodegradability of clothes is one of the latest environmental issues. Even when multiple people wear the same item of clothing for a long time, it still needs to be washed on a regular basis, which frequently causes weaning of fibres into water. These synthetic fibres, which may even reach to the scale of nanometres, are really difficult to filter and their accumulation pose a serious threat to the aquatic ecosystem and also leads to contamination of potable water4. Moreover, at every stage of clothing production, a lot of harmful waste is produced, which impacts the environment, like the use of landfills, pollution of the air and soil and inefficient use of resources5. Additionally, mass production of BC is and application BC because of expensive media and low output commercially, thereby limiting its commercial use. One of the oldest methods for producing BC is fermentation. During the tea fermentation process, BC fermentation results in the formation of cellulose-based biofilm at the air-liquid terminal. The biofilm so formed is an essential source of BC, although it is regarded a waste product as it is derived from the symbiotic culture of bacteria and yeasts (SCOBY). Researchers are examining ways to use sustainable carbon resources through bio-process refinement and minimize the price of BC production2,5.
This review aims to highlight BC as a potential alternative to bring about a revolution in the textile industry, so as to diminish the environmental stress caused by the synthetic fibres. The review also focuses on the possible low-cost production substrates for BC production and its viable applications.
Properties of Bacterial Cellulose
Cellulose, primarily found in cotton and woody plants is a superabundant polymer on the planet3,5. The textile industry makes extensive use of cellulose. Cellulose is a polymer having a chemical formula (C6H10O5)n. It has a linear chain of molecules containing Carbon, Hydrogen and Oxygen atoms (Fig. 1).
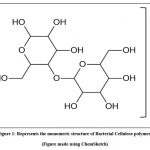 |
Figure 1: Represents the monomeric structure of Bacterial Cellulose polymer. |
The chain of β-D-glucose is not branched and is connected by β-type 1,4-glycosidic linkages. These interact with one another through the intramolecular and intermolecular hydrogen bonds. When two glucose molecules connect together, they produce the cellobiose, which is regarded as the building block of cellulose molecule. The formation of cellulose microfibrils, which contribute to the rigor of the chain and the development of straight, stable fibres, is made possible by hydrogen bonding. Due to this, the pulp has enhanced mechanical resistance and is no longer soluble in water and majority of other organic solvents6,7,8.
Since, plants are a major source of cellulose, they have been widely exploited, which has led to massive destruction of forests. Consequently, BC would be a step forward to protect the environment. In addition to this, its physio-chemical properties make it desirable for its use in the textile as well as paper industry2. Cellulose extracted from plants has also other components the most prevalent of which are lignin and hemicellulose. It also has poor crystallinity as a result of which, substantial processing is required, consuming a lot of energy, water, and toxic chemicals4. Brown was the first to report BC from the bacterium Acetobacter xylinium8. The cellulose chains are held together by hydrogen bonds that are both intra- and intermolecular that gives BC its special features like excellent purity, good water retention, low solubility, mechanical resilience, plasticity, biodegradability, biocompatibility, non-toxicity and non-allergenicity9,10.
The plant cellulose differs from the biological cellulose chiefly by its micrometric fibres, whereas the bacterial cellulose contains nano-sized fibres that are extruded through the cell wall of the bacterium10. The optical contrast between the two concerns both the appearance and the water content. The plant cellulose is fibrous, whereas the other is gel-like. When the fermentation process is immersed static, BC is found to have a 3-dimensional structure and as a result of certain properties, a high level of crystallinity is obtained for the BC (60-90%) in comparison to the plant cellulose (40%) and primarily the cotton fibres (70%)3.
The structural properties of BC depend on two factors: the origin of the strain and composition of growth media. The first determines the formation of the two distinct crystalline structure, i.e., monoclinic-I β cellulose, and triclinic-cellulose I α as it effects I α/I β. While, the latter, affects the dimension of the molecular bacterial cellulose chain. The degree of crystallinity and physicochemical properties of BC are hence determined by these characteristics3.
BC entertains many advantages over plant cellulose in terms of possessing a range of inherent physical and mechanical properties. Unlike plant cellulose, which contains hemicellulose, lignin, and pectin, BC is pure and has a polymerization degree of 4000–10,000 anhydro glucose units2. The ultrafine network of BC nano-fibres of size 3–8 nm with a high degree of uni-axial orientation makes up the 3D structure of the thick, gelatinous membrane (hydrogel sheet) that develops under static culture conditions and has a high surface area, high porosity, high crystallinity and extensive durability3,1. BC fibrils are approximately a hundred times smaller in proportions compared to plant cellulose; BC material becomes substantially more hydrophilic and has a greater capacity of holding water, as much as 100 times its own weight, when water binds to its OH groups. BC also possess substantial moldability, thickness, density, plasticity, thermochemical stability, and mechanical strength comparable to steel or Kevlar(1,2,11,12,5). Table 1 highlights the differences between bacterial cellulose (BC) and plant cellulose (PC). The BC membranes can be sterilised and are elastic and flexible3.
Table 1: Indicates the numerous structural and biological differences between Plant Cellulose (PC) and Bacterial Cellulose (BC).
| S. No. | Criterion | Plant Cellulose (PC) | Bacterial Cellulose (BC) | Reference |
| 1. | Purity | It has lesser purity as PC found in the wall of plant cells, is intimately associated with an array of hemicelluloses and lignin. | BC has very high purity as it contains no hemicelluloses, lignin, pectin or waxes. | 1 |
| 2. | Crystallinity | It has comparatively lower crystallinity (40% – 50%). | It has a higher crystallinity of over 60% – 90%. | 3 |
| 3. | Fibril structure | PC microfibrils are comparatively thicker than those of BC, leading to lower crystalline domain. | The thickness of BC nanofibrils is generally 0.1–10 mm, 100 times thinner than that of PC fibrils and is composed of glucan chains interlocked by hydrogen bonds so that a crystalline domain is produced. | 3 |
| 4. | Appearance | It has a fibrous aspect. | It has a gel-like nature. | 3 |
| 5. | Water holding capacity | It has comparatively a lower water holding capacity of about 25-35%. | Its water retention capacity is greater than 95% which is much higher than PC. | 13 |
| 6. | Biodegradability | It is comparatively difficult to degrade. | It is comparatively easier to degrade. | 14 |
| 7. | Surface Area (m2/g) | <10 | >150 | 13 |
| 8. | Porosity | It has a lower porosity of about <75%. | It is a highly porous material with porosity of about >85%, which provides high water holding capacity and also allows transfer of antibiotics into the wound, making it suitable for use in wound care. | 1, 13 |
| 9. | Pore size | 1-100 nm | 10-300nm | 14 |
| 10. | Degree of polymerization | 300-10,000 | 14,000-16,000 | 13 |
| 11. | Young’s modulus (mPa) | 25-200 | Sheet: 20,000 and single fibre: 130,000 | 13 |
| 12. | Other properties | PC generally lacks all these properties. | It has a low solubility, high mechanical resistance, elasticity, flexibility, biodegradability, biocompatibility and non-toxicity. | 3 |
| 13. | Derivative | Cotton, wood and fibers from seeds, fruits, vegetables, stalk, leaf etc. | Produced primarily by bacteria belonging to genus Agrobacterium, Gluconacetobacter, Sarcina etc. | 14 |
| 14. | Function | PC is a component of plant cell wall and is essential for survival. | BC is not essential for survival but can confer a selective survival advantage. | 15 |
The traditional methods of patternmaking and sewing can also be used to cut BC into pieces and assemble it into a garment3. In addition, animal studies have shown that BC has no teratogenic or reproductive toxicity, inflammatory reactions, or adverse effects. BC did not cause eye or dermal irritation in the primary animal model studies. Additionally, research demonstrated that BC is not mutagenic and in fact, it has been subjected to human consumption as a food for years. Surface, chemical, structural, and a variety of in situ and ex situ functionalisations have all improved BC’s properties for improved performance in a variety of applications2,16.
Recent studies indicate that in the state immediately following harvest, the wet BC sheets were extremely sturdy and unbreakable by hand pulling. In addition, they were easy to fold and had a supple feel to them. However, during testing, these samples were difficult to clamp, resulting in gradual breaks but showed an average ultimate strength of 9.71 MPa. In comparison to these, the air-dried samples produced a material that was less durable and more brittle. Probably, because the BC network’s three-dimensional structure had collapsed, these samples were relatively thin. In addition, the fabric contracted when drying, producing wrinkles and uneven widths. The latter samples, however, still seemed flexible, the material did not disintegrate when folded, and their tensile strength was much greater than that of the undried samples17.
BC is therefore, an excellent material for use in a various sectors on account of the extraordinary physical and mechanical characteristics18.
Molecular Synthesis and Bioculture Mechanism
The precise cause of cellulose formation is unknown, as different studies have opposing views. Certain bacterial strains synthesize cellulose as a defence mechanism against UV radiation, fungus, and yeasts19. Some Sarcina strains may create amorphous cellulose, which causes cells to attach to one another and aids in nutrient absorption20.
A. xylinum, also called Gluconacetobacter xylinus, is a gram-negative bacterium that can be recognized as an example for the study of cellulose production as the cellulose fibril is an exceedingly pure, metabolically inert extracellular deposit. It also possesses quick development and the capacity to be maintained under regulated circumstances. It can grow and produce cellulose from several substrates, such as hexoses, hexanoates, 3-carbon molecules like pyruvate, glycerol, and dihydroxyacetone, and 4-carbon citrate cycle dicarboxylic acids. It could polymerize approximately 200,000 glucose monomers per second. The pace of cellulose synthesis in the resting-cell system is unhindered by protein synthesis inhibitors, but is altered by the action of inhibitors/uncouplers of the electron transport chain21,22.
Mechanism Of Cellulose Synthesis in Bacteria
Uridine diphosphate (UDP)-glucose is the directly occurring sugar nucleic acid component of cellulose synthesis. The whole process beginswith glucose as a monomer till the culmination of cellulose in four enzymatic steps. It involves glucose phosphorylation via glucokinase, subsequently glucose-1-phosphate (Glc-1-P) genesis by glucose-6-phosphate (Glc-6-P) isomerisation, lastly, the formation of UDP-glucose (UDPG), and the cellulose synthase process by UDPG-pyrophosphorylase. The movement of hexose phosphate carbon to cellulose or through the pentose cycle seems to be regulated by an energy-linked mechanism, with the crossover point happening at the ATP-responsive NAD-linked glucose-6-phosphate dehydrogenase. A key stage in the formation of cellulose fibrils is the polymerization of glucose, which occurs mostly in the region immediately outside the cell surface21. (Fig. 2)
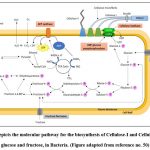 |
Figure 2: Depicts the molecular pathway for the biosynthesis of Cellulose-I and Cellulose-II using glucose and fructose, in Bacteria. |
A. xylinum typically generates cellulose I and cellulose II, two different kinds of cellulose. The former is a ribbon-like highly crystalline polymer, whereas, the latter is an amorphous polymer that is found to be more thermodynamically stable. The crystalline character of the cellulose I structure is majorly as a result of the uni-axial arrangement of the 1-4 gluco-chains with Vander Waals attraction, however for cellulose II, the 1-4 gluco-chains are placed haphazardly with greater number of hydrogen bonds. Therefore, this characteristic is crucial to thermal properties of cellulose II19.
Conditions required for growing bacterial cellulose producing strains
The properties of BC produced are influenced remarkably due to the quality of culture environment —which encompasses the bacteria’s strain, nutrition, pH level, and oxygen delivery. Static and agitated/shaking cultures are the techniques currently being used for the synthesis of BC. In contrast to the static culture strategy, which produces an asterisk-shaped, sphere-shaped, pellet-like, or irregular mass, the agitated/shaking culture method produces a mucilaginous layer of cellulose that settles on the nutrient solution surface23,24. It has been determined that acetic acid and glucose are essential nutrients for the growth of bacteria. The utilisation of acetic acid and the synthesis of gluconic acid during the earliest phases of incubation can both maintain a stable pH and fermentation environment. The bacteria may aggressively and constantly develop gluconic acid utilizing glucose provided the concentration of glucose remains greater than its acetic acid content during the fermentation process. Finally, low pH environments are inappropriate for bacterial development. According to experimental data, BC biosynthesis was stopped when the pH value dipped below the pH range of 4–7, which is necessary for BC formation. There is evidence that a rise in oxygen availability can lower BC production23.
Static Culture Method
A well-known and well-established technique for creating BC is the static culture method. This approach involves filling containers with new nutritional solution and incubating them for 1-14 days with proper temperature (28-30°C) and pH conditions (4-7). The static culture approach produces BC, which is in form of a hydrogel sheet with good structure and characteristics23. In a static culture, the cellulose-producing cells are transported to the air-liquid intersection while remaining attached to the cellulose product the cellulosic layer floats on the surface(24). After being purified with hot water and sodium hydroxide, new BC is obtained that was primrose yellow. After that, samples are thoroughly rinsed with water until the pH is neutralized, at which point the BC became white. The size of the air-liquid intersection directly influences the proportion of BC produced as the production of BC film takes place on the nutrient solution surface. Cellulose is generated in static cultures in greater amounts than in shaking cultures. The two primary issues with static culture methods are however, high cost and limited output rate23.
Agitation/Shaking Culture Method
The main concept behind the shaking culture was to optimise oxygen supply to bacteria during culture. Even while experiments revealed that not all bacterial strains could benefit from this method of increasing BC output, it escalated the process by producing BC pellicles in a variety of sizes and shapes when given the right rotation speed25. Studies reveal that a rotation speed of 100 rpm is inefficient for the process whereas an increase in speed to about 150 rpm displayed changes in the shape of BC pellicles. Raising the rotating speed had no effect on the amount of BC produced. When BC is produced in an agitated culture, its morphology and characteristics change, leading to lower levels of polymerization, a lower crystallinity index, and worse mechanical qualities23.
Production Of Bacterial Cellulose from wastes
The prohibitive price of fermentation media, which makes up approximately 30% of BC’s prime-cost, prevents it from being produced economically. The utilisation of various wastes as low-cost media has been the concern of extensive studies over the past several years with the goal of lowering this cost while also contributing to the solution of environmental issues brought on by the disposal of waste products (Fig. 3). Recycling and turning these wastes into goods with extra value, like BC, would thus be advantageous26,27.
Agro-Industry
Agriculture Industry produces a range of by-products like sweet potato mulch, bark rice, wheat grain straw, dry olive mill and corncob which are together known as plant biomass. These low-priced goods are made from resources that are renewable and accessible worldwide and are mostly made of cellulose, hemicellulose, and sometimes lignin(18). Less than ten percent of the total waste produced by this industry each day are used as substitute raw materials in other industries26.
The various sugars in corn stalk hydrolysate include acetic acid, furfural, xylose, mannose and glucose. In a study, by-products from maize stalk hydrolysis were used under ideal conditions and methodologies to show the ecological synthesis of BC. The resulting BC fibrils ranged in length from 300 nm to several micrometres and had a diameter of between 20-70 nm. Additionally, a medium for BC production can be rice bark made from agricultural residues23,2,28. By primarily undergoing acid or enzymatic hydrolysis, followed by bacterial fermentation, wheat straw can also be employed as a feedstock for the manufacture of BC26. Oat hulls, which make up almost 1/3rd of the mass of the grains and possess a cellulosic content of up to 45%, are a cheap and renewable resource. It is a global waste which is industrially sustainable and can be employed as a substrate alternative for BC synthesis 26,2.
Majority of agro-industries discard pineapple and coconut juices as waste because they are high in peptones, sugars, and trace elements. When these drinks were compared, coconut juice outperformed pineapple juice in terms of BC productivity2,29. The inedible skins of fruits and vegetables, which make up between 5-40% of the overall weight, can act as substrates for BC production since they are a substantial source of reducing sugars, essential vitamins, nutritious proteins, and many acids. For instance, orange peel can be utilized as a substrate for BC synthesis as it has 10% water content, 30–40% sugars, 15–25% pectin, around 8–10% cellulosic material and 5-7% hemi-cellulose. A. xylinum is being used in BC production to investigate other potential sources of carbon from agricultural waste, such as banana peel. Coffee Cherry Husk, a by-product of coffee cherry processing can also be employed as a potential medium for BC generation. According to the findings of the study, waste sisal is also a valuable resource for BC production30.
Beverage Industry
Daily production of large quantities of by-products remains a concern for management in the brewery and beverage industries, which strive to keep disposal costs low2. However, much of this waste can be employed as a suitable growth substrate for BC generation as it not only furnishes a low-cost method for producing BC, but it also protects the environment by preventing the waste accumulation31. A range of these by-products have been subject to studies and examined for their potential role in BC synthesis.
Sludge from makgeolli, which is frequently discarded in traditional paddy wine refineries, includes metal ions, organic acids, and nitrogen that sustain microbial growth and can therefore be used for synthesis of BC using bacterial strains like G. xylinus. Experiments have showcased the desired peaks, polymorphic structure and fibrous network of BC produced using this sludge as a substrate32,26. Revin et al. studied the utilisation of stillage (TS), cheese whey, and acidic wastes from the dairy and alcohol industries for the cost-effective production of BC using G. sucrofermentans. The study demonstrated high yield of BC with right structural characteristics and thus, suggests the use of above-mentioned by-products as an inexpensive and efficient carbon and nitrogen substrates33.
Being a plentiful supply of nutrients for the development of microorganisms, various beer industry wastes can be utilised for producing BC. Waste beer yeast is a by-product of the fermentation of different cereals and has a high nutritional content with high percentages of proteins, sugars, RNA, vitamins, glutathione and trace quantities of some metals like phosphorus, potassium, calcium, iron, magnesium, and iron. It can thus, be used as a fermentation medium for G. hansenii CGMCC 3917, where it can serve as a source of carbon and nutrients34,26. For BC production, grape bagasse, a wine production residue, was also evaluated3. Additionally, the by-products of wet maize milling are a rich supply of vitamins, nitrogen, carbon and can effectively encourage microbial fermentation and growth, thereby, presenting an appropriate substrate alternative29.
The peels and pulp of citrus fruits make up about 40-60% of their weight; are also rich in water, pectin, dietary fibres, and minerals. They also easily decompose or even become more harmful to the environment. Therefore, making BC from citrus peel and pomace via enzyme hydrolysis solution may be both economical and environmentally benign31. As by-products of the manufacture of bio-diesel, residual sugars like glucose and arabinose) and exopolysaccharides, which are produced as lipid fermentation wastes, and can also be considered as suitable substrates for BC production35. Studies also report the production of BC using Kombucha, which is a microbial consortium of different bacteria and fungi in a sweetened tea36,37. This way, utilisation of industrial by-products will not only assist the elevation of manufacturing and marketing of BC-based products, but it will also solve the brewery and beverage industry’s major waste disposal issue2.
Sugar Industry
Treacles, bagasse, and press mud are the primary sugar industry by-products which along with other by-products like factory and brewery effluents contribute significantly to pollution. Molasses have been recommended as a viable substrate media for BC generation by several A. xylinum isolates in static culture method, making the process of BC manufacture affordable, according to multiple studies carried out so far. Polyphenolic compounds are also present in molasses and majority of them share similarities with lignin in their guaiacyl and syringyl units2,38,39. Molasses have been, for a long time, used to produce a variety of industrial products, including lactic acid, polyhydroxybutyrate, ethanol, pullulan, xanthan gum, and cellulose, as a fermentation medium40. Molasses differ from sugar syrups in having a high concentration of total suspended solids and carbohydrates. They also have a low level of phosphorus, nitrogen and cysteine. The liquid sweeteners known as syrups, on the contrary, are derived from carbohydrates like maize starch and maple sap. Their high sugar content, which in maple syrup can reach 67% (weight/weight), sets them apart. Contrary to molasses, sucrose accounts for 89% of these sugars, with fructose and glucose accounting for the remaining carbohydrates. Though syrups are also used in microbial cultures, molasses are used more often18. Studies report improved physio-chemical properties of BC produced using molasses medium, with characteristic crystallinity and a high mechanical strength of about 102 ± 16.8 MPa. Pulp waste, lignocellulosic biorefinery waste, and hot water extract also make up a significant portion of the pulp mill and lignocellulosic biorefinery residual by-products consisting of mostly cellulose and hemicelluloses. Sugar, organic acids, vitamins, and minerals are also abundant in a few of them. These wastes can also be transformed into high-quality, profitably marketable products2.
Textile Industry
Cellulose polymers make up most old clothes made of cotton or regenerated cellulose (like viscose), and might be used as a cheap alternate supply of starches for the manufacturing of BC. Other kinds of textile waste that come from making yarn, fabric, or clothes could also be used, which could help cut down on BC’s production costs, save natural resources, thereby protecting the environment41.
The bulk of the organic sources utilized in the fibre and textile sector after purification and hydrolysis treatments include a lot of cellulosic material, therefore the wastes produced may be used to create a range of valuable products like BC. In a study, hydrolysate produced by enzymatic hydrolysis and pre-treatment of cotton-based textiles was used as the growth medium for the synthesis of BC2,26.
Therefore, several industrial by-products can be used for BC synthesis, which would not only commercialise the process, but also provide a sustainable alternative to waste management. A range of microbial species could be employed and grown on medium made using these wastes, according to their nutritional requirements as these industrial wastes are rich in vitamins and minerals which could act as food and energy sources for these microbes (Fig. 4).
Can BC Bring a Revolution in Textile Industry?
Textile production is one of the earliest and second-most waste producing industries in the world with improper disposal, clothing wastage, and excessive consumption becoming a phenomenon42. Cotton, though, is one of the most common textile options but 1 kg of cotton production requires almost 29 tonnes of water and a significant number of insecticides and pesticides5. Investment in disciplines like bioengineering and bio-fabrication that prospect substitutes, including the utilization of specific microbes (A. xylinum being the most efficient), to produce textiles, both for the apparel and footwear industries, would be one way to address this problem. One of the most important bioeconomy technologies is the bio-fabrication of BC. Compared to other methods of producing materials, bio-fabrication uses fewer chemicals, less water, and less energy while leaving a smaller carbon footprint42. Designers and scientists are increasingly concentrating on biomaterials like BC and its biocompatible characteristics in an effort to make the fashion business more sustainable19.
As part of her “Bio Couture” research effort, British fashion designer Suzanne Lee established the utilization of BC by experimenting with Kombucha. After that, numerous studies and experiments on BC were conducted, resulting in dyed or undyed BC artefacts, either naturally or synthetically. Using a culture substrate comprising of coconut water and factory waste, the “Malai” venture, based in British Columbia, provides vegan analogues for leather for fashion products in a range of hues43. Using static culture, researchers have recently created BC from green tea medium, as reported by Ng and Wang. They also claimed that the rigidity, flexibility, stability, and tensile strength of the BC created made it the material of choice for fashion applications19,44.
Applications Of BC in Other Industries
Cosmetology
Due to its great capability for retaining water, lack of toxicity, and lack of allergic side effects, BC is a fantastic biomaterial for the cosmetics sector45. Scientists have examined the use of the BC membrane for cosmetic purposes in addition to its medical uses and have found that the face masks made of BC, if applied for five minutes, helped to tighten the skin because the water content of the mask boosted the skin’s ability to absorb water. The bio-cellulose mask has been clinically shown to contribute to the skin’s increased moisture, thereby nourishing the skin with reduced fine lines and wrinkles. The therapeutic chemicals can also permeate deeply into the skin owing to the three-dimensional “material” formed by the interconnecting, highly absorbent fibres of bacterial cellulose. These facial masks adhere to the skin properly and were therefore proved to be beneficial for the skin with no pungent odours46.
Food Industry
Due to its exceptional ability to hold water, high purity, and dietary fibres with low caloric values, BC is a biopolymer that may be consumed47. It has also been habitually employed in the making of nata de coco, a South-East Asian native dietary fibre which is a popular munch choice for people of many countries, especially in the Philippines. It is widely manipulated in food refining owing to its chewy, sloppy, and smooth texture having few calories, little fat, and no cholesterol48,49. Apart from this, BC has been proposed as a suitable alternative as thickening and stabilising agents, low-calorie additives, surface modifiers, pale sauces and fabricated food. It is also used as an ice-cream additive, which increases the shear stress, thereby preventing ice-cream flow after melting. Due to its relevant properties, it has been reviewed ‘‘generally recognized as safe’’ (GRAS) and accepted by the FDA in 1992. It is also currently added in tofu, boiled fish pastes and is known to improve product dispersion, if used in combination with sucrose and CMC50. BC also exhibits numerous health benefits including lowering the risk of cardiovascular diseases, diabetes, obesity, treatment of gastric illnesses and as a pre-biotic51,47.
Biomedical Field
Since the 1980s, BC has been manipulated as a natural polymeric medium for nursing of injuries because it is highly biocompatible and can provide the ideal 3-D substrate for cell attachment45,52. It is also known to speed up granulation, reduce pain, and promote autolytic debridement, ensuring proper wound healing. BC nanocomposites can be used for replacement of cardiovascular tissues, artificial cornea, bone tissue engineering and dental root canal treatment. Several biomedical devices designed for cellular growth screening can also benefit greatly from the configuration of BC ultra-thin films52,53. In addition, the close contact these BC formulations have with the diseased region renders them the perfect platform for cutaneous therapeutic administration when the membranes have been fully or partially desiccated51,54.
Ecology And Paper Industry
Due to its high purity and microfibril structure, BC can be employed as a paper substrate. The paper’s surface is hydrophobic because of the more compact structure of BC. Wood-based paper has negative effects on the environment, contributes to the loss of large forests, and so on. Additionally, bacteria provide a sustainable alternative to the production of paper. Bio-packages made of BC, which is biodegradable and environmentally friendly, can lessen the impact of plastic on the environment. The highly elastic and porous filters made from bacterial cellulose indicate a bright future for wastewater treatment applications based on the bacterial pulp46. BC, a bio-polymer developed by bacterial fermentation, satisfies the criteria for a new class of highly specialized, biodegradable materials for use in environmental applications. For applications involving membrane technology, such as the filtration of heavy alloys, the catalysis of organic contaminants, the absorption of organic solvents, and the methods of oil/water separation, it has also been extensively used to support a variety of nano-particles, biopolymers, and additives55.
Bacterial Cellulose – A Sustainable Alternative Satisfying the Global SDGs
In 2015, the United Nations established the 2030 Agenda, comprising of 17 Sustainable Development Goals (SDGs) and Bacterial Cellulose production through employment of industrial waste as its substrate aims to accomplish 7 of these SDGSs, (Fig. 5) making it a perfect example of sustainable development as it can attain an appropriate balance between social, economic and environmental dimensions of growth.
 |
Figure 5: Depicts the 17 Sustainable Development Goals formalised by United Nations in 2015 and the 6 SDGs (6, 9, 12, 13, 14, 15) which can be achieved by BC production using industrial waste as its substrate. Click here to view figure |
SDG 6 focuses on the provision of clean water and sanitation. As seen in the literature, cotton, which is one of the most popular textile fabric is also one of the most water consuming and water polluting crops with an average water turnover of 4029 m3/ton. Cotton production is linked with approximately 25% of the pesticide consumption and a significant amount of water is consumed during its processing. The break out of synthetic fibres in the water systems during the course of washing further pollutes the environment. Bacterial Cellulose can thus help in combating all of these problems and can be used as a suitable and sustainable textile alternative as BC production is much more environment friendly with no use of chemicals, less water consumption, biodegradable fibres and minimum wastage42.
SDG 9 aspires to provide robust infrastructure, promote inclusive, long-term industrialisation, and support innovation; and all of this aligns well with BC production through industrial waste utilization as substrate. Biotextiles is a new age innovation harbouring cleaner processes which favour industrial scale-ups via sustainable development directives42,56.
BC production could also address SDG 12 with the goal of ensuring sustainable production and consumption habits. It strives to significantly reduce waste creation through trash avoidance, reduction, recycling, and reuse. Consequently, the virtue of biodegradability is underlined; hence, biotextiles are not regarded environmentally hazardous and may even be disposed in composters. Microbial fermentative chemical and material production from regenerative resources can help SDG 12 both ecologically and economically42,57.
Synthetic textile manufacture emits huge amounts of greenhouse gases and depletes fossil fuel and water resources. Moreover, dangerous and poisonous substances are used in their manufacture. In comparison, BC production is far more bio-economically sustainable, requiring less land, water, and energy. Even a minor commercialization of BC as a leather alternative might result in less demand for animal hides, less greenhouse gas emissions, and less tanning-related toxicity, thus addressing one of the most important – SDG 1356.
SDG 14 (Life under water) and SDG 15 (life on land) could also be addressed through implementation of biotextile production. Not only does the synthetic fibres and micro-plastics affect the marine life but their production directly or indirectly affects the life on land as well; PC too leads to destruction of plant life. Microbial BC production can therefore reduce the problem of water pollution and land mitigation56, 57.
Lastly, it can be suggested that the textile manufacturing system should be reformed because it is still incompatible with both environmental and social concerns. As a result, considering new and more sustainable materials, such as bacterial cellulose, is a type of mitigation that is in line with the environmental interest and global SDGs42.
Policies to Endorse Bacterial Cellulose Production
The government can play an important role in promoting the adoption of bacterial cellulose as a sustainable material for clothing. By implementing policies that encourage research, provide tax incentives, set procurement policies, promote education and awareness, provide subsidies, and regulate production and use, the government can help to create a market for sustainable materials and accelerate their adoption. Here are some policy recommendations:
Promoting research and development: The government can invest in research and development of bacterial cellulose and other sustainable materials. This could involve funding for academic institutions, research centres, and private companies that are working on developing new materials.
Tax incentives: The government could provide tax incentives for companies that use sustainable materials in their production processes. This could encourage more companies to switch to sustainable materials, as they would be able to save money on taxes.
Procurement policies: The government can set procurement policies that prioritize the use of sustainable materials in government purchases. This could create a market for sustainable materials, which could help drive down costs and increase adoption.
Education and awareness campaigns: The government can launch education and awareness campaigns to promote the use of sustainable materials. This could include advertising campaigns, public service announcements, and educational materials aimed at consumers and businesses.
Subsidies: The government can provide subsidies to companies that are using sustainable materials. This could help to offset the higher costs associated with these materials, making them more competitive with traditional materials.
Additionally public education can be a powerful tool for promoting the adoption of bacterial cellulose and other sustainable materials/clothes. By raising awareness, educating consumers, highlighting sustainable brands, encouraging clothing swaps, and engaging schools and universities, public education can help to drive demand for sustainable materials and accelerate their adoption. Here are some ways in which public education can be used to promote sustainable materials:
Raising awareness: Public education can raise awareness about the environmental impact of traditional materials, such as cotton and polyester. This can be done through advertising campaigns, public service announcements, and educational materials that highlight the benefits of sustainable materials and the negative impacts of traditional materials.
Educating on production processes: Public education can help to educate consumers about the production processes of sustainable materials. This can include information about how bacterial cellulose is produced and the environmental benefits of these processes. This can be done through educational materials, videos, and interactive exhibits.
Highlighting sustainable clothing brands: Public education can also highlight sustainable clothing brands that use bacterial cellulose and other sustainable materials in their products. This can be done through social media campaigns, influencer marketing, and collaborations with sustainable brands.
Encouraging sustainable clothing swaps: Public education can also encourage sustainable clothing swaps, where consumers can exchange their old clothes for sustainable options. This can be done through local events and online communities.
Engaging schools and universities: Public education can engage schools and universities to promote the use of sustainable materials. This can include integrating sustainable materials into school curriculums, hosting sustainability workshops, and encouraging student-led initiatives to promote sustainable materials.
Mitigation of Potential Risk and Ethical Consideration
Bacterial cellulose has the potential to be a sustainable and eco-friendly alternative to traditional materials in the clothing industry. However, as with any new material, there are potential risks and ethical considerations that need to be mitigated. Here are some key areas of concern and possible mitigation strategies:
Environmental impact: The production of bacterial cellulose involves the cultivation of bacteria in large tanks of nutrient-rich liquid. This process requires a significant amount of water, energy, and other resources. To mitigate this, alternative substrate such as that obtained from agro-industry, beverage industry and sugar industry as discussed above.
Contamination: Bacterial cellulose production can be vulnerable to contamination by other bacteria or fungi. Production facilities can mitigate this risk by implementing strict hygiene protocols, using sterile equipment, and monitoring the production process closely.
Ethical considerations: There are ethical considerations associated with the use of bacterial cellulose, such as the use of genetically modified bacteria. To mitigate this, companies can use non-GMO bacteria or develop sustainable production methods that do not require the use of genetically modified organisms.
Social impact: The adoption of bacterial cellulose could potentially have a significant impact on traditional textile industries and communities. Companies can mitigate this by engaging with local communities and providing support for sustainable economic development.
Hence, the application of bacterial cellulose for textile production necessitates rigorous evaluation of possible dangers and ethical issues. Companies may contribute to making the use of bacterial cellulose sustainable and advantageous for all parties involved by establishing adequate safety standards, minimising environmental effect, resolving ethical issues, and interacting with local communities.
Conclusion and Future Prospects
In conclusion, bacterial cellulose (BC) presents an exciting opportunity to revolutionize the textile industry by providing a sustainable and eco-friendly alternative to synthetic fibres and Plant Cellulose. The use of BC in the textile industry can significantly reduce the environmental stress caused by synthetic fibres, addressing several of the United Nations’ Sustainable Development Goals.
The potential applications of BC are diverse, ranging from clothing to non-woven fabrics, and can also be modified to meet specific requirements, making it an attractive option for many industries. As the world becomes more conscious of environmental issues, the demand for sustainable materials is likely to increase, and BC can play a significant role in meeting this demand. Although BC production is still limited by its high production cost, ongoing research aims to reduce its cost by using sustainable carbon resources and refining the bio-process.
Therefore, future prospects include development of techniques and methods for the development of this biotechnology-based polymer which encourage a shift to a cleaner, greener, renewable and scalable economy(58). In addition, to promote the adoption of BC as a sustainable material, governments can implement policies that encourage research, provide tax incentives, set procurement policies, promote education and awareness, provide subsidies, and regulate production and use. Public education can also be a powerful tool for promoting the adoption of BC and other sustainable materials. Overall, the use of BC in the textile industry presents a potential revolution in sustainable and eco-friendly manufacturing, and it is exciting to see the possibilities that this biopolymer can offer. As research continues and production costs decrease, BC has the potential to become a widely used alternative to synthetic fibres in the near future, contributing to a more sustainable and environmentally friendly world.
Acknowledgement
The authors would like to thank the management of Shaheed Rajguru College of Applied Science for Women, University of Delhi, for providing facilities for carrying out the present study.
Conflict of Interest
I hereby declare that all the authors and the corresponding author do not have any conflict of interest.
Funding Sources
There is no external source of funding for conducting the research.
References
- Ashjaran A., Yazdanshenas M. E., Rashidi A., Khajavi R., Rezaee A. Overview of bio nanofabric from bacterial cellulose. Journal of the Textile Institute. 2013;104(2):121-131.
CrossRef - Hussain Z., Sajjad W., Khan T., Wahid F. Production of bacterial cellulose from industrial wastes: a review. Cellulose. 2019;26(5):2895-2911.
CrossRef - Costa A. F. S., Rocha M. A. V., Sarubbo L. A. Bacterial cellulose: an eco-friendly biotextile. International Journal of Textile and Fashion Technology. 2017;7:11-26.
- Kamiński K., Jarosz M., Grudzień J., Pawlik J., Zastawnik F., Pandyra P., Kołodziejczyk A. M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose. 2020;27(9):5353-5365.
CrossRef - Provin A. P., dos Reis V. O., Hilesheim S. E., Bianchet R. T., de Aguiar Dutra A. R., Cubas A. L. V. Use of bacterial cellulose in the textile industry and the wettability challenge—a review. Cellulose. 2021;28(13):8255-8274.
CrossRef - Santos S. M., Carbajo J. M., Quintana E., Ibarra D., Gomez N., Ladero M., Eugenio M. E., Villara J. C. Characterization of purified bacterial cellulose focused on its use on paper restoration. Carbohydrate Polymers. 2015;116:173-181.
CrossRef - Ul-Islam M., Khan T., Park J. K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydrate Polymers.2012;88:596-603.
CrossRef - Brown R. M., Saxena I. M. and Kudlicka, K. Cellulose biosynthesis in higher plants. Trends in Plant Science.1996;1:149-56.
CrossRef - Pecoraro É., Manzani D., Messaddeq Y., Ribeiro, S. J. Bacterial cellulose from Glucanacetobacter xylinus: preparation, properties and applications. In Monomers, polymers and composites from renewable resources. Elsevier. 2007;369-383.
CrossRef - Donini Í. A. N., Salvi D. T. B. DE, Fukumoto F. K., Lustri W. R., Barud H. S., Marchetto R., Messaddeq Y., Ribeiro S. J. L. Biossíntese e recentesavançosnaprodução de celulosebacteriana. Eclética Química.2010;35:165-178.
CrossRef - Klemm D., Kramer F., Moritz S., Lindström T., Ankerfors M., Gray D., Dorris A. Nanocelluloses: a new family of nature‐based materials. AngewandteChemie International Edition. 2011;50:(24), 5438-5466.
CrossRef - Lee K. Y. (ed.): Nanocellulose and sustainability: production, properties, applications, and case studies. CRC Press. 2018.
CrossRef - Coseri S. Insights on cellulose research in the last two decades in Romania. Polymers. 2021;13(5):689.
CrossRef - Naomi R., Bt Hj Idrus R., Fauzi M. B. Plant-vs. Bacterial-derived cellulose for wound healing: A review. International journal of environmental research and public health. 2020;17(18):6803.
CrossRef - Augimeri R. V., Varley A. J., Strap J. L. Establishing a role for bacterial cellulose in environmental interactions: lessons learned from diverse biofilm-producing Proteobacteria. Frontiers in microbiology. 2015;6:1282.
CrossRef - Badshah M., Ullah H., Khan A. R., Khan S., Park J. K., Khan T. Surface modification and evaluation of bacterial cellulose for drug delivery. International journal of biological macromolecules. 2018;113:526-533.
CrossRef - Elsacker E., Vandelook S., Damsin B., Van Wylick A., Peeters E., De Laet L. Mechanical characteristics of bacterial cellulose-reinforced mycelium composite materials. Fungal biology and biotechnology.2021;8(1):1-14.
CrossRef - Velásquez-Riaño M., Bojacá V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose. 2017;24(7):2677-2698.
CrossRef - Rathinamoorthy R., Kiruba T. Bacterial cellulose-A potential material for sustainable eco-friendly fashion products. Journal of Natural Fibers. 2022;19(9):3275-3287.
CrossRef - Li Y., Yang Q., Liu B., Zhang Q., LiuY., Zhao X., Li S. Improved water dispersion and bioavailability of coenzyme Q10 by bacterial cellulose nanofibers. Carbohydrate Polymers. 2022;276:118788.
CrossRef - Ross P., Mayer R., Benziman M. Cellulose biosynthesis and function in bacteria. Microbiological reviews. 1991;55(1):35-58.
CrossRef - Saxena I. M., Brown R. M. Biosynthesis of bacterial cellulose. Bacterial nanocellulose: a sophisticated multifunctional material. 2012;1-18.
CrossRef - Wang J., Tavakoli J., Tang Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydrate polymers. 2019;219:63-76.
CrossRef - Rani M. U., Appaiah A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Annals of microbiology. 2011;61(4):781-787.
CrossRef - Hu Y., Catchmark J. M. Formation and characterization of sphere like bacterial cellulose particles produced by Acetobacter xylinum JCM 9730 strain. Biomacromolecules. 2010;11(7):1727-1734.
CrossRef - Ul-Islam M., Ullah M. W., Khan S., Park J. K. Production of bacterial cellulose from alternative cheap and waste resources: a step for cost reduction with positive environmental aspects. Korean Journal of Chemical Engineering. 2020;37(6):925-937.
CrossRef - Ul-Islam M., Wajid Ullah M., Khan S., Kamal T., Ul-Islam S., Shah N., Kon Park J. Recent advancement in cellulose-based nanocomposite for addressing environmental. Recent patents on nanotechnology. 2016;10(3):169-180.
CrossRef - Cheng Z., Yang R., Liu X., Liu X., Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresource technology. 2017;234:8-14.
CrossRef - Kongruang S.: Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. In Biotechnology for Fuels and Chemicals. Humana Press. 2007;763-774.
CrossRef - Kadier A., Ilyas R.A., Huzaifah M.R.M., Harihastuti N., Sapuan S.M., Harussani M.M., Azlin M.N.M., Yuliasni R., Ibrahim R., Atikah M.S.N., Wang J. Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (BC) production: Mechanism, advances, and future perspectives. Polymers. 2021;13(19):3365.
CrossRef - Fan X., Gao Y., He W., Hu H., Tian M., Wang K., Pan S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydrate Polymers. 2016;151:1068-1072.
CrossRef - Hyun J. Y., Mahanty B., Kim C. G. Utilization of Makgeolli sludge filtrate (MSF) as low-cost substrate for bacterial cellulose production by Gluconacetobacter xylinus. Applied biochemistry and biotechnology. 2014;172(8):3748-3760.
CrossRef - Revin V., Liyaskina E., Nazarkina M., Bogatyreva A., Shchankin M. Cost-effective production of bacterial cellulose using acidic food industry by-products. brazilian journal of microbiology. 2018;49:151-159.
CrossRef - Lin D., Lopez-Sanchez P., Li R., Li Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresource Technology. 2014;151:113-119.
CrossRef - Vazquez A., Foresti M. L., Cerrutti P., Galvagno M. Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. Journal of Polymers and the Environment. 2013;21(2):545-554.
CrossRef - Domskiene J., Sederaviciute F., Simonaityte J. Kombucha bacterial cellulose for sustainable fashion. International Journal of Clothing Science and Technology. 2019:31(5):644-652.
CrossRef - Avcioglu N. H., Birben M., Bilkay I. S. Optimization and physicochemical characterization of enhanced microbial cellulose production with a new Kombucha consortium. Process Biochemistry. 2021;108:60-68.
CrossRef - Poddar P. K., Sahu O. Quality and management of wastewater in sugar industry. Applied Water Science. 2017;7(1):461-468.
CrossRef - Keshk S., Sameshima K. The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Applied Microbiology and Biotechnology. 2006;72(2):291-296.
CrossRef - Cakar F., Özer I., Aytekin A. Ö., Şahin, F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydrate Polymers. 2014;106:7-13.
CrossRef - Hong F., Guo X., Zhang S., Han S. F., Yang G., Jönsson L. J. Bacterial cellulose production from cotton-based waste textiles: enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresource Technology. 2012;104:503-508.
CrossRef - Provin A. P., Cubas A. L. V., Dutra A. R. D. A., Schulte N. K. Textile industry and environment: can the use of bacterial cellulose in the manufacture of biotextiles contribute to the sector?Clean Technologies and Environmental Policy. 2021;23(10):2813-2825.
CrossRef - da Silva C. J. G., de Medeiros A. D., de Amorim J. D. P., do Nascimento H. A., Converti A., Costa A. F. S., Sarubbo, L. A. Bacterial cellulose biotextiles for the future of sustainable fashion: a review. Environmental Chemistry Letters. 2021;19(4):2967-2980.
CrossRef - Ng F. M., Wang P. W. Natural self-grown fashion from bacterial cellulose: a paradigm shift design approach in fashion creation. The Design Journal. 2016;19(6):837-855.
CrossRef - Song J. E., Kim, H. R. Bacterial cellulose as promising biomaterial and its application. In Advances in textile biotechnology. Woodhead Publishing. 2019;263-277.
CrossRef - Niyazbekova Z. T., Nagmetova G. Z., Kurmanbayev A. A. An overview of bacterial cellulose applications. Eurasian Journal of Applied Biotechnology. 2018;(2).
CrossRef - Gregory D. A., Tripathi L., Fricker A. T., Asare E., Orlando I., Raghavendran V., Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Materials Science and Engineering: R: Reports. 2021;145:100623.
CrossRef - Esa F., Tasirin S. M., Abd Rahman N. Overview of bacterial cellulose production and application. Agriculture and Agricultural Science Procedia. 2014;2:113-119.
CrossRef - Mohite B. V., Patil, S. V. A novel biomaterial: bacterial cellulose and its new era applications. Biotechnology and Applied Biochemistry. 2014;61(2):101-110.
CrossRef - Lin S. P., LoiraCalvar I., Catchmark J. M., Liu J. R., Demirci A., & Cheng K. C. Biosynthesis, production and applications of bacterial cellulose. Cellulose. 2013:20(5):2191-2219.
CrossRef - Blanco Parte F. G., Santoso S. P., Chou C. C., Verma V., Wang H. T., Ismadji S., Cheng, K. C. Current progress on the production, modification, and applications of bacterial cellulose. Critical reviews in biotechnology. 2020;40(3):397-414.
CrossRef - Rajwade J. M., Paknikar K. M., Kumbhar J. V. Applications of bacterial cellulose and its composites in biomedicine. Applied microbiology and biotechnology. 2015;99(6):2491-2511.
CrossRef - Picheth G. F., Pirich C. L., Sierakowski M. R., Woehl M. A., Sakakibara C. N., de Souza C. F., Martin A. A., da Silva R., de Freitas R. A. Bacterial cellulose in biomedical applications: A review. International journal of biological macromolecules. 2017;104:97-106.
CrossRef - Lustri W. R., Barud H. G. O. B., Barud H. D. S., Peres M. F., Gutierrez J., Tercjak A., De Oliveira O. B., Ribeiro, S. J. L. Microbial cellulose—biosynthesis mechanisms and medical applications. Cellulose-Fundamental Aspects and Current Trends. 2015;1:133-57.
CrossRef - Urbina L., Corcuera M. Á., Gabilondo N., Eceiza A., Retegi A. A review of bacterial cellulose: sustainable production from agricultural waste and applications in various fields. Cellulose. 2021;28(13):8229-8253.
CrossRef - García C., Prieto M. A. Bacterial cellulose as a potential bioleather substitute for the footwear industry. Microbial Biotechnology. 2019;12(4):582.
CrossRef - Jang W. D., Hwang J. H., Kim H. U., Ryu J. Y., Lee S. Y. Bacterial cellulose as an example product for sustainable production and consumption. Microbial Biotechnology. 2017;10(5):1181.
CrossRef - Schiros T. N., Antrobus R., Farías D., Chiu Y. T., Joseph C. T., Esdaille S., … & Lu H. H. Microbial nanocellulose biotextiles for a circular materials economy. Environmental Science: Advances. 2022;1(3):276-284.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.







