Manuscript accepted on : 16/12/2019
Published online on: --
Plagiarism Check: Yes
Reviewed by: Hanan Abd Elhafeez
Second Review by: Hassan Hassan Elsayyad
Final Approval by: Dr Philip D. Cotter
Bartholomew Chukwuebuka Nwogueze1, Anthony Emeka Ojieh1*, Richard Nduka Ossai1  , Chidinma Nwanneamaka Eke2
, Chidinma Nwanneamaka Eke2  and Stephen Chibueze Ufearo2
and Stephen Chibueze Ufearo2
1Department of Human Physiology, Delta State University, Abraka, Delta State, Nigeria.
2Department of Human Physiology, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria.
Corresponding Authors Email: tonniojie@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2804
ABSTRACT: Background: Medicinal plants are increasingly getting recognition. Cissus aralioides is a lofty climber belonging to the family of Vitaceae and widely distributed in South-Eastern region of Nigeria. Varied therapeutic benefits as well as well health harming effects have been reported about the plant. This study evaluated the effects of aqueous extract of Cissus aralioides leaf on reproductive functions in female Wistar rats.Methods: 72 adult female Wistar rats weighing between 110-180/g were used for the study. The animals were randomly grouped into 6 groups of twelve rats each (n=12). Groups 1 and 2 served as control while groups 3 to 6 were the experimental groups and were administered (100/mg, 200/mg, 300/mg and 500/mg)/kg of the extract respectively for 4 weeks. At the end of the experiment, blood samples were collected and centrifuged to obtain the serum for biochemical analysis for Follicle Stimulating Hormone (FSH), Estrogen and Luteinizing Hormone (LH). Data were analyzed using the SPSS package and expressed as mean± SEM and ANOVA.Results: There was significant body weight reduction across groups administered with the extract. Also, there were significant decrease in estrogen level in groups that received 300/mg/kg and 500/mg/kg when compared with the control groups, a similar decrease was not observed with LH and FSH across the groups.Conclusion: Observations from this study shows that aqueous leaf extract of Cissus aralioides decreases circulating levels of estrogen in female Wistar rats.
KEYWORDS: Reproductive Hormones; Cissus Aralioides; Estrogen; FSH; LH
Download this article as:| Copy the following to cite this article: Nwogueze B. C, Ojieh A. E, Ossai R. N, Eke C. N, Ufearo C. S. Reproductive Function Evaluation in Female Wistar Rats Treated with Aqueous Leaf Extract of Cissus Aralioides. Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Nwogueze B. C, Ojieh A. E, Ossai R. N, Eke C. N, Ufearo C. S. Reproductive Function Evaluation in Female Wistar Rats Treated with Aqueous Leaf Extract of Cissus Aralioides. Biosci Biotech Res Asia 2019;16(4). Available from: https://bit.ly/306rJ6V |
Indroduction
Background
Fertility as a concept is the most important aspect of human life, which is not just an expression of miracle and mystery but directly has a possible effect on the future of an organism and procreation (1). Despite the fact that the current growth rate is adding-up on daily basis, about 78 million people every year has been estimated to have reproductive issues relating to infertility, hence, accounting for the incidence of global health problem worldwide (2). A study conducted by the World Bank revealed that poor reproductive health is the major cause of death and disability especially among women of 15 – 49years in developing countries (3).
The normal physiological principle guiding female reproductive efficiency involves an interplay between the hypothalamus that secretes gonadotropin-releasing hormone (GnRH) in a pulsatile manner and the pituitary gland which is stimulated by the hypothalamus via the hypothalamo-pituitary-adrenal axis to regularly the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), in addition to the ovary that has both methodical enzymatic system and steroidogenesis for producing sex steroid hormones such as; estrogen and progesterone alongside a functional uterus that responds to these hormones (4).
Plants have attracted global concern considering their value across various cultures in traditional medicine for primary health care. According to Ifeanyi et al., (2011) 70-80% of the world’s population depends on medicinal practice as rightly reported by the World Health Organization. Ethnobotanical studies in recent times are seen as the most viable method for characterization and quantification of new medicinal plants as well as refining and refocusing on trends of plants with bioactive components (6). The use of medicinal plants has continually played a vital role in remediating fertility related problems. This accounts for the reason why in Nigeria there has been a paradigm shift away from the use of the synthetic drug among different strata of people (7).
Cissus aralioides Planch is a medicinal climber plant belonging to the family of Vitaceae. The plant is covered with irritating hairs and red saps that tends to be acidic (8). In Nigeria, Cissus aralioides is popularly called “Eriri agwo” Ugu ofia” and “Agbarandunenu” in Igbo language while it is referred to as “Abekama marun” in Yoruba language and “Dodoriga” or “Hada rawake” in Hausa Language; whereas the common name is “monkey plum” (9). C. aralioides seeds, leaves, stem, and roots according to Assob et al., (2011) have been employed in treatment of cuts or injuries, microbial contaminations or infections.
Reports suggest that at the biological and molecular levels, estrogenic activity can be mimicked by phytoestrogens which are plant chemicals that promote estrogenicity. This implies that plant estrogen has potency for inhibiting estrogen and progesterone therefore resulting in an alteration the reproductive physiology of the female leading to fertility problems. James et al., (2013) affirmed that such interactions could either act as agents or antagonists thereby either increasing or decreasing endogenous hormone level in the body. Furthermore, FitzGerald and Dinan, (2008) affirmed that estradiol stimulates the efficient development of the uterine lining, thickening during pre-ovulatory phase of menstrual cycle as well as growth, proliferation, and development of reproductive organs of experimental animals; in addition, stimulates secondary characteristics in synergy with other hormones such as; FSH and LH through the action of gonadotropin-releasing hormones (GnRH).
The motivation to carry out this study comes from the fact that in south-eastern regions of Nigeria, this plant is commonly used for various therapeutic purposes and in soup preparation. Also, in this same region, evidences of fertility problems are on the increase especially amongst married couples. Though there are various pathogenesis of infertility, the increase in different society has already been observed by several national and international organizations.
Methods
Some of the materials used include; blending machine (SBM-2977, Osaka, Japan), measuring cylinders (varying volumes; 20ml, 50ml, 500ml) and universal specimen containers, digital weighing balance (Model JA 2003), sieve, UV-VIS spectrophotometer (model 752N), micropipettes (REMI), ELISA microplate (HIPO MPP-96, BIOSAN), electrical thermostatic water bath boiler (model DK 420), and microscope (light digital).
Preparation of Plant Extracts
The collected Leaf of C. aralioides were air-dried in shade for a period of 4weeks, thereafter pulverized with a blinder, soaked in distilled water for 24hours and passed into a sieve (ASTM-E11, RETSCH, Germany) of mesh size 0.25/mm; then the resulting rhizome powder with a constant weight of 500g was extracted using the cold maceration filtration method, the filtrate was then concentrated with Soxhlet apparatus and the resultant extract was stored in fridge at 40C to prevent it from losing its potency. The different concentrations were prepared from the stock.
Animal Handling
Ninety (90) healthy adult female Wistar rats of 12-14/weeks old weighing between 110-180/g were purchased from the animal house unit of Delta State University, Abraka and transported in a plastic cage to Nnamdi Azikiwe University, Awka, the site of the experimentation. The animals were allowed a period of 10-14/days for acclimatization and during this period, they were allowed uninhibited access to food and water. They were fed with standard growers mash diet in their respective cages. The experiment was carried out in accordance with the U.K Animals (Scientific Procedure) Act, 1986 and associated guidelines, EU Directives 2010/63/EU for animal experiments. Also ethical approval was sought and gotten from Ethical Committee of Nnamdi Azikiwe University for use of animals for experiment.
Drug/Extract Administration
The hormone drug used was conjugated β-estrogen, manufactured and supplied by Medipharm (Prt) Ltd. 108, Kotlakphat Industrial Estate Lahore, Pakistan. The drug (0.625/mg) was diluted in distilled water and the constituted stock administered to the standard control group. The drug extracts were administered orally daily for a period of four (4) weeks; however, the control group received an equivalent volume of normal saline. The experimental rats were grouped as shown in Table 1 below:
Table 1: Extract Administration and Grouping
| Categories | Treatment |
| Group 1 | Normal saline (Normal Vehicle Control): untreated with Conjugated β-estrogen or the extract. |
| Group 2 | Standard drug (Conjugated β-estrogen) (0.625/mg) |
| Group 3 | 100/mg/kg Cisus aralioides extract |
| Group 4 | 200/mg/kg Cisus aralioides extract |
| Group 5 | 300/mg/kg Cisus aralioides extract |
| Group 6 | 500/mg/kg Cisus aralioides extract |
Biochemical Aanalysis
At the end of the 4th week of administration, 10 rats each were selected randomly from each group and blood were collected using ocular puncture into sterile containers, spinning and stored in a fridge. Thereafter, the rats were sacrificed through cervical dislocation. The serum was used to assay for Follicle Stimulating Hormone (FSH), Estrogen and Luteinizing Hormone (LH) levels across the various groups using ELISA microplate (HIPO MPP-96, BIOSAN) following the protocol and procedure provided on the accubind kit from Biocode, Belgium.
Histological Examination
At the end of the experiment, samples of ovaries and uterus from all groups of rats were histologically prepared, following the method described by Bancroft and Gamble, (2002) for fixation, tissue processing, staining, and photomicrography.
Statistical Analysis
Data(s) generated were statistically analyzed using the Statistical Package for Social Sciences (SPSS) software version 20.0. The results were expressed as mean ± SEM. Comparisons were made between groups using ANOVA. Values of less than 0.05 were considered statistically significant.
Results
The results on body weight changes showed that there was a significant decrease in body weight of the Wistar rats administered with plant leave extract of Cissus aralioides when compared with the normal control and standard control, however, this decrease in body weight was not concentration dependent as rats administered 300/mg/kg.wt had the highest level of decrease in body weight followed by 100/mg/kg.wt, 500/mg/kg.wt, and 200/mg/kg.wt. The standard drug had a significant body weight gain when compared to the control as shown in Table 2 below:
The result of the mean and standard error of mean for the reproductive hormone levels for estrogen, FSH and LH are shown in Table 3. The result revealed that there was no significant decrease in the estrogen levels of the female Wistar rats administered with standard drugs, 100/mg/kg and 200/mg/kg of plant extract when compared to the control group. However a statistically significant decrease in estrogen level was observed in the group administered with 300/mg/kg and 500/mg/kg of the extract respectively compared to the control and the standard. The decrease in LH was not significant and there were no observed decrease in FSH in the same groups.
There were no significant difference was observed in the surface follicles and corpus luteum of control, standard and extracts treated rats as there was the presence of corpus luteum and various stages of follicular development in all the groups. A subjective observation of the histo-architecture of the ovary and uterus showed no significant changes across all the groups.
Table 1: Extract Administration and Grouping
| Categories | Treatment |
| Group 1 | Normal saline (Normal Vehicle Control): untreated with Conjugated β-estrogen or the extract. |
| Group 2 | Standard drug (Conjugated β-estrogen) (0.625/mg) |
| Group 3 | 100/mg/kg Cisus aralioides extract |
| Group 4 | 200/mg/kg Cisus aralioides extract |
| Group 5 | 300/mg/kg Cisus aralioides extract |
| Group 6 | 500/mg/kg Cisus aralioides extract |
Table 2: Change in Body Weight of Wistar Rats Treated with Cissus Aralioides
| Parameters | Weight (g) | % Weight Change | |
| Initial | Final | ||
| Normal Control | 115.0±6.45 | 133.33±7.11 | 15.90 |
| Standard
|
124.16±9.65* | 132.08±711 | 6.37 |
| 100mg/kg | 172.50±7.29*a | 159.92±6.87* | -7.29 |
| 200mg/kg | 172.50±3.28*a | 170.00±6.15*a | -1.45 |
| 300mg/Kg | 170.00±4.92*a | 152.92±8.45 | -10.05 |
| 500mg/kg | 189.58±3.51*a | 177.50±6.86*a | -6.37 |
Values are expressed as mean ± SEM. *p<0.05 when compared with the control, a p<0.05 compared with standard
Table 3: Reproductive Hormone Indices of Aqueous Extracts of Cissus Aralioides on Wistar Rats
| Parameters | Estrogen
(pg/ml) |
LH
(mIU/ml) |
FSH
(mIU/ml) |
| Normal Control | 165.66±17.12 | 4.64±.02 | 2.23±0.03 |
| Standard | 126.30±10.48 | 4.68±0.02 | 2.28±0.03 |
| 100/mg/kg | 111.40±19.45a | 7.46±2.84c | 2.26±0.03 |
| 200/mg/kg | 138.06±26.29a | 7.51±2.74c | 2.43±0.16 |
| 300/mg/Kg | 74.14±20.65ab | 4.74±0.02 | 2.24±0.05 |
| 500/mg/kg | 56.74±13.80ab | 4.79±0.02 | 2.33±0.03 |
All values are expressed as Mean ± SEM, values sharing superscript ‘a’ indicate significant decrease when compared to the control, ‘b’ indicates a significant decrease when compared to the standard; ‘c’ indicates significant increase when compared to the control and, ‘d’ indicate a significant increase when compared to the standard (p<0.05).
Discussion
The results of the present study showed a significant decrease in body weights of extract treated groups, especially at higher doses, although these decreases in body weights were not concentration dependent. A significant decrease in mean body weight has been reported as an important index of toxicity (14). There was observed reduction in the feeding habits of animals in the treated groups as the extract administration continued, this could suggests that the extract suppressed appetite. However, there was gain in weight in animals administered with conjugated estrogen compared with the control group and treated groups..
Results from the biochemical study show that aqueous leaf extracts of Cissus aralioides have dose-dependent effects on the estrogen levels of the female Wistar rats. There was a statistically significant decrease in estrogen level in animals administered with 300mg/kg and 500mg/kg groups when compared to the control and the standard drug groups. Same observation was not the case with LH and FSH levels (see Table 3). This could be linked to the assertion of Koneri et al., (2006) and Amah et al., (2012) that the pituitary-gonadal axis is important for the maintenance of the reproductive system, thus, any distortion to this axis can be deleterious.
Considering the fact that estradiol is known to be essential for embryonic and fetal development as noted by Mitak et al., (2001). Hence, estrogen antagonists and inhibitors of estrogen synthesis present in plant extracts interfere with placental function and cause abortions (18). The declining estrogen levels at higher doses as observed in this study may be due to an inhibitory effect of C. aralioides on pituitary gonadotropins and direct toxic effect on follicular and theca cells in the female Wistar rats. According to Amah et al., (2012) and Ladan et al., (2013) such suppression could ultimately result in alterations in the functional integrity of the uterine cycle as well as other hormone interaction and secretion.
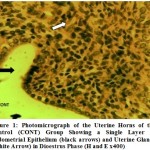
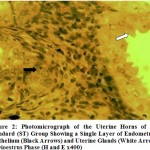
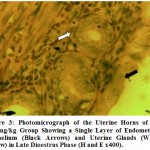
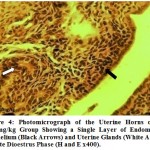
Histologically, it was observed from Figure 3 – 12 the present study that there was no significant difference in the uterus and ovary of female Wistar rats across the treated groups when compared to the control (see figure 1), and standard drug group (see figure 2). The uterine histology showed normal features: a single-layered columnar epithelial cell with elongated nuclei at the base of the cells, highly folded epithelial lining, numerous and tortuous endometrial glands and edematous stroma. Consequently, there was no observable pathologic change in the ovary histology; the ovary showed normal cortical stroma containing ovarian follicles and corpus luteum in various stages of development and an inner medullar comprising richly vascularized loose connective tissue. This is consistent with the assertion of Don (2007) that the corpus luteum usually produces progesterone which helps to maintain the pregnancy if the oocyte is fertilized by the spermatozoa. This is an indication that the plant extract had no histological alterations on the estrous cycles of the Wistar rats.
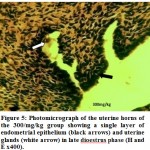
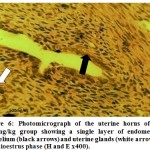 |
Figure 6:Photomicrograph of the uterine horns of the 500/mg/kg group showing a single layer of endometrial epithelium (black arrows) and uterine glands (white arrow) in late dioestrus phase (H and E x400). |
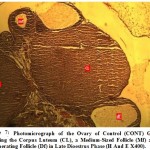 |
Figure 7: Photomicrograph of the Ovary of Control (CONT) Group Showing the Corpus Luteum (CL), a Medium-Sized Follicle (Mf) and a Degenerating Follicle (Df) in Late Dioestrus Phase (H And E X400). |
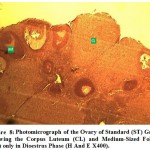 |
Figure 8: Photomicrograph of the Ovary of Standard (ST) Group Showing the Corpus Luteum (CL) and Medium-Sized Follicle (Mf) only in Dioestrus Phase (H And E X400). |
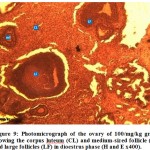 |
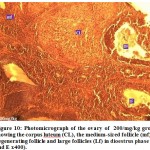
Figure 9: Photomicrograph of the ovary of 100/mg/kg group showing the corpus luteum (CL) and medium-sized follicle (mf) and large follicles (LF) in dioestrus phase (H and E x400). |
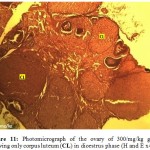 |
Figure 11: Photomicrograph of the Ovary of 300/mg/kg Group Showing only Corpus Luteum (CL) in Dioestrus Phase (H and E x400). |
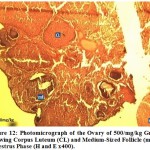 |
Figure 12: Photomicrograph of the Ovary of 500/mg/kg Group Showing Corpus Luteum (CL) and Medium-Sized Follicle (mf) in Dioestrus Phase (H and E x400). |
Conclusion
Conclusively, observations from this study suggest that C. aralioides when administered orally in high dosage, has anti-fertility potentials and the mechanism of action could be either by inhibition of the pituitary responsiveness to GnRH altering the normal sequence of secretion of LH and FSH or by directly inhibiting the ovarian response to circulating LH and FSH, leading to reduced production and release of estrogen. The mechanism of action of this plant makes it a potential natural source of of synthesizing anti-fertility drugs. This can be a useful addition to already existing medications for family planning especially in rural settings.
References
- Hoffman, L.D. 2012. Fertility & Contraception.pp.1-4.
- United Nations, 2010. Department of Economic and Social Affairs population Division. World Population Prospects 1-2.
- World Bank, 2011. Maternal mortality and morbidity: Health, nutrition and population, Bulletin of the World Health Organization; 84(3): 204-10
- Iptisam, I.M. and Gokalp, O. 2012. “Sex Hormones and Infertility”. Erciyes University / Department of Obstetrics and Gynecology Turkey. Pp 1-19
- Ifeanyi, P.O., Ihemdirim, C.U.O., Victor, U.O. and Idorenyin, F.E. 2011. The potentiality of medicinal plants as the source of new contraceptive principles in males, North American Journal of Medical Sciences; 3(6): 255-263.
- Khafagi, I.K. and Dewedar, A. 2000. The efficiency of random versus ethno-directed research in the evaluation of Sinai medicinal plants for bioactive compounds, Journal of Ethnopharmacology; 71: 365-376.
- Sofowora, E. 2008. “Medicinal Plants and Traditional Medicines in African”; University of Ife Press, Nigeria.
- Ezeja, M.I., Omeh, Y.N., Onoja, S.O. and Ukaonu, I.H. 2015. Anti-inflammatory and Antioxidant Activities of the Methanolic Leaf Extract of Cissus aralioides. American Journal of Pharmacological Sciences; 3(1): 1-6.
- Adelanwa, M. A. and Haruna, H. B. 2013. Survey of some plants found in gurara local government area of Niger state, Nigeria: International Journal of Applied Biological Research: 5(1): 43-54
- Assob, J.C., Kamga, H.L., Nsagha, D.S., Njunda, A.L., Nde, P.F., Asongalem, E.A., Njouendou, A.J., Sandjon, B. and Penlap, V.B. 2011. Antimicrobial and toxicological activities of five medicine plant species from Cameroon Traditional Medicine”, BMC Complementaryand Alternative Medicine.; 11(1): 70-75.
- James, A., Rogers, L., Metz, V. and Wee, Y. 2013. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms; Journal of Molecular Immunology; 53: 421-430
- FitzGerald, P. and Dinan, T.G. 2008. Prolactin and dopamine: What is the connection? Journal of Psychopharmacology; 22(1): 12-19.
- Bancroft, J.D. and Gamble, M. 2002. “Theory and practice of Histological Techniques”; Churchill Livingstone, Edinburgh. 16-64.
- Raza, M., Al-Sabanah, O.A., El-Hadiyah, T.M. and Al-Majed, A.A. 2002. Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss Scientia Pharmaceutica 70: 135 – 145.
- Koneri, R., Balaraman, R. and Saraswati, C.D. 2006. Antiovulatory and abortifacient potential of the ethanolic extract of roots ofMomordica cymbalaria Fenzl in rats. Indian Journal of Pharmacology; 38(2):111–114.
- Amah, C.I., Yama, O.E. and Noronha, C.C. 2012 Infecund evaluation of cycling female Sprague-Dawley rats: an aftermath treatment withMomordica charantia seed extract. Middle East Fertility Society Journal. 17(1):37–41.
- Mitak, K., Gojmerak, T., Mandic, B. and Cvetinic, Z. 2001. Changes in serum concentration of 17β-estradiol during estrous cycle after treatment with atrazine, Veterinary Medicine; 5: 145 – 148
- Okereke, C. and Onuoha, S. 2015. Effect of Ethanolic Extract ofCannabis sativa on Progesterone and Estrogen Hormones in Female Wistar Rats. Journal of Reproductive System and Sex Disorder; 4:150
- Ladan, K., Elmira, H., Arefeh, M. and Shahin, A. 2013. Knowledge, attitude and practice of herbal remedies in a group of infertile couples.Acta Medica Iranica; 51(3):189–194

This work is licensed under a Creative Commons Attribution 4.0 International License.