Manuscript accepted on : 25-May-2019
Published online on: 10-06-2019
Plagiarism Check: Yes
Reviewed by: Mishra Rosalin
Second Review by: Psolano
Final Approval by: Dr. Haseeb Ahmad Khan
Antimicrobial Properties of Rosa Indica (A New Start with Nature)
Deepika Pathak and Kruti M. Dave* and Luluaa Aliasgar
Mehsana Urban Institute of Sciences, Ganpat University.
Corresponding Author E-mail: davekruti2@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2755
ABSTRACT: Plants have been played an important role as a resource of natural medicines for human health, from the long period of time. Some plants show antimicrobial properties. The most important merit of using plant derived medicines is that they are low priced, readily available and showing minor side effect. The present study deals with the antibacterial potential of crude extracts of petals of Rosa indica. Through agar disk diffusion method the antimicrobial potential of Rosa indica was examined. Petals were collected, dried and its crude extract was obtained. Methanol and acetone were used as the extraction solvent. These extract were examined against two gram-positive (Staphylococcus aureus, Bacillus cereus) and two gram-negative (E-coli, Salmonella typhi) bacteria. Extracts prepared by all solvents showed antimicrobial action and established zone(s) of inhibition. By performing phytochemical tests we have observed positive results for flavanoid, tannin and alkaloid. These phytochemical have capability to inhibit the growth of microorganism or can fight against microorganism. Rich source of compounds have been obtained in this particular plant. For inhibition of multiple drug resistant microorganisms, this extract can be tested. For making herbal drugs, extract of Rosa indica may prove to be useful as an advance step in future.
KEYWORDS: Antimicrobial Potential; Crude Extract; Phytochemical Test; Rosa Indica; Zone of Inhibition
Download this article as:| Copy the following to cite this article: Pathak D, Dave K. M, Aliasgar L. Antimicrobial Properties of Rosa Indica (A New Start with Nature). Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Pathak D, Dave K. M, Aliasgar L. Antimicrobial Properties of Rosa Indica (A New Start with Nature). Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2KBJWDH |
Introduction
In developing countries to cover basic health needs Plant materials is widely used for the treatment of diseases. Diseases in human can be caused by many types of Pathogens (1). Bacterial contamination especially members of Gram-negative bacteria like Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa are associated with food poisoning reports (2). Also other causal agents of food borne disease are Gram-positive bacteria including Bacillus cereus and Staphylococcus aureus (3). B. cereus is can cause serious and potentially fatal non-gastrointestinal-tract infection (4). S.aureus can cause a range of illnesses, from minor skin infections, such as carbuncle, pimple folliculitis, abscesses, and etigogo skin syndrome to life-threatening diseases such as sepsis, bacteremia, toxic shock syndrome, pneumonia, meningitis, endocarditis and osteomyelitis. It is still one of the five most common causes of hospital-acquired infections and is often the cause of wound infections following surgery. This diseases may be treated with antibiotics, but there is concern that widespread use of antibiotic might lead to antibiotic resistance. (5). Because of the appearance of bacterial resistance to antimicrobial agents more effort is being made to find alternative antimicrobial components, so rather than synthetic products natural products are more preferred (6). Antimicrobial substances are those which kill or inhibit the growth of microorganism such as protozoa,fungi,bacteria. All species of life including bacteria can produce antimicrobial substance which contains antimicrobial protein; they enhance the immunity by stimulating adaptive immune system (7).
Antimicrobial activity is shown by a variety of plant materials and phytochemicals (8, 9). Plants produce phytochemicals which help microorganisms generally to thrive or thwart competitors, predators, or pathogens. The name phytochemical has been arrived from the Greek word phyton, meaning plant. In traditional medicines phytochemicals has been used. For maintaining human health plants are playing a major role. Plants containing antimicrobial compounds have been found to possess antimicrobial activity. To develop drugs India has rich traditionally using medicinal plants. According to world health organization [WHO] any plant having substances that can be used for therapeutic healing of chemo pharmaceutical semisynthetic new drug is reoffered as medicinal plant(10). Plants provide us natural products as a new agents for antimicrobial use. Secondary Metabolites are special feature of higher plants having capacity to produce a large number of organic chemicals of high structural diversity. Because of fewer side effects; we use herbal anti-microbial agents.
The Present investigation deals with the study of antimicrobial activity of Rosa indica, having genus Rosa within the family Rosacea has perennial flower shrub or vine, that contains over 100 species and comes in a variety of colors. From a long period of time roses have been one of the worlds most popular ornamental plants. The size, shape and color of the flower varies(11). Rosacea contains an extensive range of phytochemicals.(12, 13).
In the Indian system of medicine, As a vehicle for other medicines various rose preparations are used as an mild laxative, astringent, tonic, antibacterial agents, enlarged tonsils, treatment of sore throat and gall stones, for cooling effect(14). For digestive tract disorders, impact of rose oil has been found positive (15). In a previous study, levoglucosan (5.69%), pyrogallol (21.92%), 5-hydroxymethylfurfural (11.52%), quinic acid (43.12%) and 4H-pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl (8.31%) were the major identified components in methanolic extract of rosa indica petals(16). Intention of this study was to evaluate the antimicrobial activities and phytochemical screening of rosa indica against staphylococcus aureus, bacillus cereus and salmonella typhi.
Materials and Method
Microorganism
There are three microorganisms were used to assess the antibacterial properties two of three organisms are gram-positive bacillus cereus, staphylococcus aureus and one is gram-negative Salmonella typhi.
Plant materials
Petals of Rosa indica.
Solvent system for Extraction
In this study two solvent systems were used for plant extraction these are methanol and acetone.
Preparation of Plant Extracts
To study antimicrobial potential of medicinal plants, it is very necessary to extract chemical components of our interest. The step includes preliminary washing, drying, and crushing of petals in the process for obtaining equivalent sample. Maximum contact should be maintained between the surface of sample and solvent to enhance the kinetics of extraction. During the extraction procedure, all the potentially active components must be maintained.
The plant material i.e. petals of rosa indica were brought, then dried it under shade and by using mortar and pestle dried petals were crushed to form powder and stored in an air tight plastic container until used. For testing the potential of medicinal plants sample were prepared in methanol and acetone solvents. Extracts were prepared by dissolving 20gm of fine powder in 100ml methanol and acetone. The contents were incubated for 48 hours. Using soxhlet extraction method respective extracts were obtained. Then concentrated to dryness, residues obtained were preserved at 4ºC. For further in-vitro studies of antimicrobial activity these extract were used (17).
Antibacterial Activity
For primary selection of the therapeutic agent disk diffusion method is used. The test microorganisms were inoculated and media was poured in to plate. 1 gm of crude extract were dissolved in 50 ml of DMSO.(18). Then after saturate disk with plant extract,and it was placed on Petri plate containing Media. For the maximum growth of the microorganism these plates were incubated at 37˚C for one to two days. surrounding the disc clear, distinct zone of inhibition was observed. The diameter of zone of inhibition was measured, which was expressed in millimeter (mm) and compared with the standard. (19).
Phytochemical Analysis
Extract and powdered specimens were screened for the presence of phytochemicals. And respective phytochemicals were identified.
Test for Tannins
About 1ml of extract was dissolved in 3ml of water and were placed on water bath for 5 minutes and then filtered. 1ml ferric chloride mixed to the filtrate. Dark green color and blue- black color if formed that will indicates that the presence of tannins(20).
Test for Flavonoids
To remove the fatty materials i.e. lipid layer, 0.5 gm of extract was mixed with petroleum ether and shaked. The obtained residue was added in 20ml of alcohol and filtered. 3 ml of the filtrate was dissolved with 4 ml of 1 % potassium hydroxide. A dark yellow color observed which indicates the positive result for flavonoid.
Test for Alkaloids
0.5 to 0.6 gm. of various extract was added in 8 ml of 1 % HCL, heated and filtered. 2 ml of the filtrate were mixed separately with both reagent (Mayer and Wagners). Presence of alkaloid can be detected from its turbidity or precipitate formation.
(Mayers reagent:- Add 1.36 gm of HgCl2 in 60 ml water and 5 gm of KI in 10 ml of water mix both and add sufficient water to make 100 ml. this will give cream or -pale yellow precipitate indicating the positive result (21).
Wagners reagent:- Mix 2 gm of iodine and 6 gm of KI in 100 ml water. That give brown or reddish brown precipitate indicate the desirable result.(22)).
Result
The results showed that the extracts screened against two gram-positive bacteria bacillus cereus and staphylococcus aureus, and gram-negative salmonella typhi showed petals of Rosa indica using agar disk diffusion method shows that rosa indica possessed bactericidal properties. Table 1 shows zone of inhibition against pathogens and table 2 shows presence of phytochemicals. While figure 1 shows photographs of antibiogram of rosa indica.
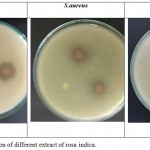 |
Figure 1: Zone of inhibition of different extract of rosa indica.
|
Table 1: Zone of inhibition of different extract of rosa indica in millimeter.
| Extract | B.cerus | S.aureus | S.typhi |
| Methanol | 20mm | 15mm | 6mm |
| Acetone | 14mm | 11mm | 3mm |
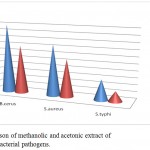 |
Figure 2: Comparison of methanolic and acetonic extract of R. indica against bacterial pathogens.
|
Table 2: Phytochemical constituent analysis of methanol and acetone extracts of R. indica. Where (+) = Present and (-) = absent; High amount = (+++); Relatively high = (++), Trace amount = (+).
| Phyto-constituent | Solvent system | Plant extract |
| Phenolic compounds | Methanol | +++ |
| Acetone | ++ | |
| Alkaloids | Methanol | +++ |
| Acetone | ++ | |
| Tannins | Methanol | +++ |
| Acetone | ++ | |
| Flavonoids | Methanol | ++ |
| Acetone | + | |
| Xantho proteins | Methanol | – |
| Acetone | – | |
| Saponins | Methanol | – |
| Acetone | – |
Discussion
Considering that the globe is facing a growing variety of multidrug-resistant microorganisms, varied studies are conducted so as to pick out new compounds, like those from natural resources that area unit of very importance (23). Natural product (secondary metabolites) from medicative plants might doubtless management microbic growth and area unit a supply of the many potent and powerful medicine. The remedies supported these plants typically have stripped-down facet result (24).
Antimicrobial activity of Rosa was performed against Bacilli caryophylloid dicot genus, staphylococci aureus, and Salmonella typhi. The pathogenicity of B. cereus, whether or not enteric or nonintestinal, is intimately related to tissue-destructive/reactive exoenzyme production .Among these secreted toxins :four hemolysins (25). Three distinct phospholipases, AN emesis-inducing poisonous substance, and 3 pore-forming enterotoxins: haemolysin BL (HBL), nonhemolytic cytotoxin (NHE), and toxin K (26, 27, 28) diarrheagenic within the duct may be a nonhemolytic three-component cytotoxin, selected NHE(29). Also S. aureus produces many molecules that additionally contribute to the formation of abscesses. Such molecules embrace those who recruit neutrophils, cause host cell lysis, and are concerned within the formation of the protein capsule close the symptom(30).Whereas, infective salmonellae eaten in food survive passage through the internal organ acid barrier and invade the mucous membrane of the little and huge viscus and turn out toxins (31).
Plants are full with range of secondary metabolites, such as, terpenoids, tannins, flavonoids and alkaloids which is found to have good antimicrobial potential. Flower extract holds phenolics mixtures like tannins that are actually good antimicrobial compounds (32). The activity is attributed to their ability to complicated with additional cellular and soluble proteins and with microorganism semipermeable membrane (33). The restrictive impact conjointly is also attributable to the presence of phenol through manufacturing chemical element bonds with proteins, that converts its structure and blocks supermolecule synthesis (34).
Literature survey reveals that many studies were printed on anti-fungal, antibacterial drug and anti-oxidant activities of roses(35). The physiological functions of acne rosacea is also partly as a result of the abundance of flavonoids and synthetic resin compounds, additionally called bioactive agents(36). synthetic resin compounds retain a good vary of bio-chemical activities, like freeradical scavengers, anti-bacterial, antioxidants( 37, 38, 39 ), anti-inflammatory(40), antimutagenic(41) and anticancer(42). Like synthetic resin compounds, flavonoids additionally show inhibitor activities, they were ascertained to guard deoxyribonucleic acid against aerophilous damages(43, 44). Beside that they possess sturdy resistance against ultraviolet radiation radiation having wavelength of 254 nm and probably it’s believed that they may be employed in anti-solar creams (45). in a very shell medicative plants square measure the foremost vital supply for sorts of medication as a result of phytochemicals gift in these plants square measure a lot of precise, atmosphere friendly, simply analyzable. The hydrophobic nature of plant extracts allows them to partition lipids of the microorganism cytomembrane and mitochondria, troubling cell structures and rendering them a lot of semipermeable. intensive run from microorganism cells or the exit of important molecules and ions ends up in death (46).
The current study exposes the antimicrobial result of methanol and acetone extracts of hibiscus rosa against different microorganisms. The plant exhibits antibacterial potential and produced zone(s) of inhibition. Because of the variety and complexity of the usual mixtures of bioactive mixtures in the crude plant extract, it is somewhat difficult to define every compound present and explain its structure in a solitary study.
Thus phytochemical offer high-class platform for biological purposes and structural variation which are important for drugs finding, their biological possessions support in dropping the risk of various long-lasting disease.(47, 48)Further investigations is necessary for separation and description of active elements of the extracts and also to explicate the mechanism of their achievement.
Acknowledgements
This work would have not been possible without the support of Dr. Amit Parikh, Principal, MUIS. ,Dr Priti Patel, HOD of Biotechnology and Mr Nehal Rami, HOD of Microbiology. I am grateful to all of those with whom I have had the pleasure to work during this research project.
References
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell.New York: Garland Science; 2002; 4th edition; 0-8153-4072-9
- N. Solomakos, A. Govaris, P. Koidis, N.Botsoglou. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage Meat Sci. in Laboratory of Hygiene of Foods of Animal Origin, 2008; 80; pp. 159-166.
- L.C. Braga, J.W. Shupp, C. Cummings, M. Jett,J.A. Takahashi, L.S. Carmo. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production by J. Ethnopharmacol. 2005; 96, pp. 335-339.
- Bottone EJ. Clin Microbiol Rev. Bacillus cereus, a volatile human pathogen in Division of Infectious Diseases,Clin Microbiol Rev. 2010; 382-98.
- Aiysha Thompson, Dilruba Meah, and Paula E Row. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome.The official journal of the International Society for Complementary Medicine Research (ISCMR)2013; 13:338
- Hindumathy C.K Umesh kumar, kunal kumar study of antimicrobial activity of Rosa indica against gram positive and gram negative microorganisms. International Journal of Microbiology Research 2012; 4; 3; 186 – 189
- JurenkaJ Therapeutic applications of pomegranate (Punica granatum L.): a review,Alternative Medicine Review, 2008; vol. 13, no. 2, pp. 128144.
- Hidayathula S, Chandra KK, Chandrashekhar KR, Phytochemical evaluation and antibacterial activity of pterospermum diversifolium blume. Int J Pharma Pharma Sci. 2011; 3 (2): 165 167.
- Yanagida, A., T. Kanda, M. Tanabe, F. Matsudaira, and J. G. O. Cordeiro, Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutants streptococci. J. Agric Food Chem.2000; 48:56665671.
- Hassanali, An investigation of Antimicrobial Compounds for Immunomodulating and Antiadhesion properties. Immunology and Infectious Disease and research Laboratory, PhD Thesis, 2003; 01-186.
- R P Mishra, Mohammad Arshad and Abdul Sami. Antibacterial Properties of Rosa indica (L.) Stem, Leaves and Flowers by 1R&D Division, MRD LifeSciences, Lucknow, Uttar Pradesh, India. 2 Integral University, Lucknow, Uttar Pradesh, India.2011; 2230 – 7885
- Olsson ME, Andersson S, Werlemark G, Uggla M, Gustavsson KE. Carotenoids and phenolics in rose hips in Acta Horticulturae, 2005; 490:249-53.
- Uggla M, Gustavsson KE, Olsson ME, Nybom H.Changes in colour and sugar content in rose hips (Rosa dumalis L. and R.) International Journal of Fruit Science 2011; 14:1, pp 28-41.
- Singh V., Kaul V. K., Singh B., Sood R. P.. Damask rose (Rosa damascenaMill.): cultivation and processing. In: Handa S. S., Kaul M. K., editors.Supplement to Cultivation & Utilization of Aromatic Plants. Jammu Tawi, India: RRL; 1997; 195.
- Boskabady M. H., Shafei M. N., Saberi Z., Amini S. Pharmacological effects of Rosa damascena in Iranian Journal of Basic Medical Sciences.2011; 14(4):295307.
- Bai S., Seasotiya L., Malik A., Bharti P., Dalal S. Bioactive compounds and pharmacological potential of Rosa indica L. and Psidium guajava L. methanol extracts as antiurease and anticollagenase agents in Der Pharmacia Lettre; 2015; 7(1):179184.
- Wang, L and C.L Weller, Recent Advances in extraction of Nutraceuticals from plants. Trend in food science and technology (Elsevier), 2006; 17: 300-312.
- El‐Mahmood, A.M., Doughari, J.H. and Chanji, F.J. ‘Invitroantibacterial activities of crude extracts of Nauclealatifolia and Daniellaoliveri’.Sci. Res. Essay;2008; Vol.3 no.3 102‐105.
- Vander, B.D.A. and Vlietrick. Screening methods for antibacterial and antiviral agents from higher plants. In: Assay for Bioactivity. K. Hostiettman (eds.). Academic Press, London. 1999; 47-69
- Edeoga HO. Phytochemical constituents of some Nigerian medicinal Plants. Afr J Biotechnol;2005; 4:6858.
- Evans WC, “Treaseand Evans Pharmacognosy”, Harcourt Brace and company. Asia pvt. Ltd.Singapore, 1997.
- Wagner.H. Pharmazeutische Biologic, 1993; 5 th Edition, AUFI.15 BN 3-437-20 498-X.
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Geneva.2014; 978 92 4 156474 8
- Swadha A, Debasisa M. USA: IGI Global. Computational methods for identification of novel secondary metabolite biosynthetic pathways by genome analysis. In: Handbook of research on computational and systems biology: Interdisciplinary applications;2011; pp. 380381.
- Granum, P. E. Bacillus cereus and its toxins. By J. Appl. Bacteriol. Symp. Suppl. 1994; 76:615-665.
- Lund, T., and P. E. Granum. Comparison of biological effect of two different enterotoxin complexes isolated from three different strains of Bacillus cereus. By Microbiology 1997; 143:3329-3336.
- Lund, T., M.-L. DeBuyser, and P. E. Granum. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol; 2000; 38:254-261.
- Saki, C., T. Iuchi, A. Ishii, K. Kumagai, and T. Takagi. Bacillus cereus brain abssesses occurring in severely neutropenic patients: successful treatment with antimicrobial agents, granulocyte colony-stimulating factor, and surgical drainage. 2001; Int. Med. 40:654-657.
- Lund, T., and P. E. Granum. Comparison of biological effect of two different enterotoxin complexes isolated from three different strains of Bacillus cereus. Microbiology 1997; 143:3329-3336.
- Scott D. Kobayashi, Natalia Malachowa, and Frank R. DeLeo. Pathogenesis of Staphylococcus aureus Abscesses in the american journal of pathology, cellular and molecular biology of disease.2014; 185(6): 1518–1527.
- Giannella RA. Salmonella. In: Baron S, editor. Medical Microbiology,4th edition. Galveston (TX): University of Texas Medical Branch at Galveston.1996; Chapter 21; ISBN-10: 0-9631172-1-1
- Scalbert AC. Antimicrobial properties in tannins. American Journal of Plant Sciences, Vol.8 No.7, Phytochem.1991; 30:38753883.
- Ionela DC, Ion IB. Plant products as antimicrobial agents. Secţiunea Genetică Biologie Molecula. 2007;8:104–111.
- Khder. Effect of Allium sativum and Myrtus communis on the elimination of antibiotic resistance and swarming of Proteus mirabilis. A. K. Jordan Biol. Sci., 2008; 1(3):124-128.
- Basim E, Basim H. Antibacterial activity of Rosa damascena essential oil.Fitoterapia 2003; 74:394396.
- Wen D, Li C, Di H, Liao Y, Liu H. Auniversal HPLC method for the determination of phenolic acids in compound herbal medicines. J Agric Food Chem 2005; 53(17):6624-9.
- Leenen R, Roodenburg AJC, Tijburg LBM, Wiseman. A single dose of tea with or without milk increases plasma antioxidant activity in humans. European Journal of Clinical Nutrition 2000; 54(1):87-92.
- Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sciences 2000; 66(8):709-23.
- Jafari M, Zarban A, Pham S, Wang T. Rosa damascena decreased mortality in adult Drosophila. J Med Food; 2008; 11(1):9-13.
- Crespo ME, lvez J, Cruz T, Ocete MA, Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Medica; 1999; 65(7):651-3.
- Miyazawa M, Okuno Y, Nakamura SI,Kosaka H. Antimutagenic activity of flavonoids from Pogostemon cablin. J Agric Food Chem; 2000; 48(3):642-7.
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L.Flavonoids: Promising anticancer agents.Medicinal Research Reviews 2003; 23(4):519-34.
- Pawlak, K., W. Bylka, B. Jazurek, I. Matlawska, M. Sikorska, H. Manikowski and G. Bialek-Bylka. Antioxidant activity of flavonoids of different polarity, assayed by modified abtscation radical decolorization and epr technique, Acta Biol. Cracoviensia Series Botanica,2010; 52: 97-104.
- Kalim, M.D., D. Bhattacharyya and A. Banerjee. Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unanisystem of medicine, BMC Complement Altern. Med. 2010; 16: 10:77.
- Tabrizi, H., S.A. Mortazavi and M.Kamalinejad. An in vitro evaluation of various Rosa damascene flower extracts as a natural antisolar agent, Int. J. Cosmet. Sci.2003; 25: 259-265.
- Rastogi, R.P. and Mehrotra, B.N. Glossary of Indian medicinal plants. by National Institute of science communication,New Delhi, India, 2002; 20-25.
- J.A. Khan and S. Tewari, . A Study on Antibacterial Properties of Rosa indica against Various Pathogens, Asian Journal of Plant Science and Research; 2011; 1 (1): 22-30.
- Verpoorte R, Contin A and Memelink, J. Biotechnology for the production of plant secondary metabolites Phytochemistry Reviews, 2002; Volume 1, Issue 1, pp 13–2.

This work is licensed under a Creative Commons Attribution 4.0 International License.





