Manuscript accepted on : 14-May-2019
Published online on: 24-05-2019
Plagiarism Check: Yes
Reviewed by: Armando Zepeda Bastida
Second Review by: Fernando Lidon
Sony Kumari*1, Rabbul Ibne A. Ahad1,2, Mobina Ahmed1, Dhanapriya Moirangthem1 and Drishtirupa Phukan1
1Department of Biotechnology, University of Science and Technology Meghalaya, Baridua - 793101, Meghalaya, India.
2Department of Biochemistry, North-Eastern Hill University, Shillong - 793022, Meghalaya, India.
Corresponding Author E-mail: sonykumari_15@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2760
ABSTRACT: The present study investigated the effects of cooking temperature on the biochemical characteristics, antioxidant and antimicrobial activity of three different seeds of Citrus fruits (Citrus limon, Citrus limetta, Citrus maxima and Citrus aurantifolia) collected from Assam, India. Total soluble sugar (72 mg/mL) were highest in Citrus maxima hydro 2-propanol seed extract before heating and 50 mg/mL in Citrus limon hydro-methanol, Citrus limetta hydro-methanol and Citrus maxima hydro-methanol seed extract after heating. Total soluble proteins before and after heating were highest 82 mg/mL and 88 mg/mL in Citrus limon 2 propanol seed extract. Free amino acid contents before and after heating were highest (62 µg/mL in Citrus limon hydro-propanol) and (40 µg/mL in Citrus limon hydro-methanol) and free fatty acids were 29.2 µg/mL and 23 µg/mL in Citrus maxima methanol extract, respectively. H2O2 scavenging activity before and after heating were highest in Citrus aurantifolia propanol (59%) and in Citrus limon (67%), respectively. Total antioxidant capacity was found highest in Citrus maxima hydro propanol (92.5%) before heating and in Citrus aurantifolia 2-propanol (65%) after heating. Antimicrobial activity of the seed extracts was studied on B. subtilis, E. coli and P. aeruginosa and minimum inhibitory concentration of the four Citrus fruits was determined. MIC of the different seed extracts was observed for 100% (v/v), 75% (v/v), 50% (v/v) and 25% (v/v) against three test microbes viz. Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli. For Bacillus subtilis, the MIC was found to be at 100%, 100%, 75%, and 75% to the extract of Citrus aurantifolia hydro 2-propanol, Citrus limon methanol, Citrus limetta hydro 2 propanol and Citrus maxima hydro 2-propanol, respectively. For E. coli, the MIC was found to be at 100%, 75%, 100% and 100% for Citrus aurantifolia hydro 2-propanol, Citrus limon hydro 2-propanol, Citrus maxima hydro 2-propanol and Citrus limetta hydro 2 propanol, respectively. For Pseudomonas aeruginosa, the MIC was found to be at 100%, 100%, 75% and 50% for Citrus aurantifolia methanol, Citrus limon methanol, Citrus maxima hydro 2-propanol and Citrus limetta hydro 2 propanol, respectively.
KEYWORDS: Antimicrobial Potential; Antioxidant Activity; Biochemical Characteristics; Citrus Fruits; Hydro 2 Propanol; Seed Extracts
Download this article as:| Copy the following to cite this article: Kumari S, Ahad R. I. A, Ahmed M, Moirangthem D, Phukan D. Effect of Cooking Temperature on Biochemical Characteristics, Antioxidant Activity and Antimicrobial Potential of Seed Extracts of Assam, India. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Kumari S, Ahad R. I. A, Ahmed M, Moirangthem D, Phukan D. Effect of Cooking Temperature on Biochemical Characteristics, Antioxidant Activity and Antimicrobial Potential of Seed Extracts of Assam, India. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2X9gYP2 |
Introduction
Citrus fruits are biochemically important because of great medicinal properties Hammer et al., (1999). The genus Citrus belong to family Rutaceae and sub family Aurantiodeae. It is believed that most of the species under genus Citrus are a native to tropical and sub-tropical regions of South East Asia including India where the availability is predominant. From Indian counterpart, North-East India and almost in all the region of South-East Asia are considered to be the Centre of origin and diversity of Citrus species Borthakur (1992); Kumari et al., (2016). Citrus fruit is extensively used by juice processing industries while the peels are disposed as waste material. The juice yield from any Citrus fruit is less than half of the fruit weight and as a result massive amounts of wastes (peels, fibre and seeds) are generated every year around the world. In general, peels, seeds and pulps (> 50% of the fruit) are dealt with as wastes, while potentially; they are being used as a source of valuable daily used products El-Adawy et al., (1999). The species of the fruit are found to be of medicinal values and are also used in confectionary, in perfume industry and toiletry. Hence, the Citrus waste can be very useful as they used as medicine for its pharmacological properties to counter different diseases and thereby generate revenues. There has been a recent surge of studies on the chemical composition (fatty acid content in particular) of the oil of seeds of different species of Citrus. El-Adawy et al., (1999 revealed that the Citrus seeds and its flours were rich in oil and protein contents. Both Citrus seeds and flours proved to be a good source of minerals, e.g. K, Ca, P, Na, Fe and Mg. In many studies, the measured oil content of Citrus seeds: Tunisian Citrus seeds (26–36%) Saıdani et al., (2004), Brazilian Rangpur lime seeds (32–38%) Reda et al., (2005). Citrus fruits have been valued as part of a nutritious and tasty diet. The flavors provided by Citrus are among the most preferred food additive in the world and it is evident from the popularity of using by the people because of adding good taste and nutritional value to the food. It is also well established that the Citrus and Citrus products are rich sources of vitamins, minerals and dietary fiber (non-starch polysaccharides) that are very essential for growth and development. Dietary guidelines and recommendations encourage the consumption of Citrus fruits and their products lead to widespread nutritional benefits across the population. Citrus is the most commonly found source of vitamin C. However, like most other whole foods, Citrus fruits also contain an impressive list of other essential nutrients including glycemic and non-glycemic carbohydrate (sugars and fibre), potassium, folate, calcium, thymine, niacin, vitamin B6, phosphorous, magnesium, copper, riboflavin, panthothenic acid and a variety of phytochemicals. In addition, Citrus c ontains no fat or sodium and being a plant food, no cholesterol. The average energy value of fresh citrus is also low which can be very important for consumers concerned about putting on excess body weight.
As an important food, it has antioxidant properties and its nutrients prevent or reduce oxidative damage to our bodies. These agents are able to remove the deleterious effects of free radicals within our body. Now a day, considerable focus is on the development and evaluation of natural antioxidants and free radical scavengers plant materials which are rich in poly-phenolic compounds. It is well known that plant derived poly-phenols have remarkable antioxidant and free radical scavenging activity adding multiple nutritional, physiological and pharmaceutical benefit to the humans. Extracts prepared from peel, flowers and leaves of bitter orange (Citrus aurantium L.) are popularly used in order to minimize central nervous system disorders Pultrini et al., (2006). Reactive oxygen species (ROS) collectively refer to both the oxygen free radicals (the oxygen superoxide and the hydroxyl radical) and some non-radical (hydrogen peroxide) derivatives of oxygen Shoji et al., (2008) are generated as products normal metabolism in the body Zhang et al., (2009).
In general, bacteria have the genetic ability to transmit and acquire resistance to drugs which are utilized as therapeutic agents Gislene et al., (2000). The essential extracts, oils and other plants products have evoked interest as sources of natural antioxidant and antimicrobial parameters. They have been screened for their potential uses as alternative remedies for the treatment of many infectious diseases Tepe et al., (2004). Essential oils are more effective in controlling biofilm cultures due to their better diffusibility and mode of contact Al-Shuneigat et al., (2005). For a long period of time, plants have been a valuable source of natural products for maintaining human health. The use of plant extracts and phytochemicals, both with known antimicrobial properties, can be of great significance in therapeutic treatments Seenivasan et al., (2006). The antimicrobial activities of Citrus plants, oil and extracts were investigated from many years. The antimicrobial abilities of essential oils among which Citrus oils are also shown to be a particularly interesting field for applications in the food and cosmetic industries Caccioni et al., (1998). Hammers et al., (1999) studied the effect of essential oils from C. aurantium, C. limon, C. paradisi and many other plant oils and extracts found that the minimum inhibitory concentrations (MIC) were between 5-2% (v/v). AL- Jedah et al., (2000) analyzed the action of combined spices including lemon in its mixture and found that the spices mixture was able to exert static effect on all assayed bacteria. Hayes and Markovic (2002) investigated the antimicrobial properties of lemon against Staphylococcus aureus, Klebsiella, Escherichia coli, Pseudomonas aeruginosa and Candida albicans.
This study detailed the biochemical analysis such as total soluble sugar, total soluble protein, total amino acid and free fatty acid of the seed of four different Citrus fruits isolated from Sonapur and Pitonipara of Assam, India and their H2O2 scavenging activity and total antioxidant capacities were measured. These seeds contained antimicrobial properties and MIC of the different extracts were determined.
Materials and Method
Sample Collection
The fully matured and ripen fruits of Citrus limon and Citrus limetta were collected from Sonapur of Kamrup district, Assam as well as Citrus aurantifolia and Citrus maxima were collected from Pitonipara of Nalbari district of Assam, India (Fig. 1). A dozen of each fruit sample was collected from the two sites and brought to the laboratory for the experimental study.
 |
Figure 1: Collection sites of the Citrus fruits are presented in the above figure – Citrus limon (lemon), Citrus limetta (mosumi), Citrus aurantifolia (lime) and Citrus maxima (robab).
|
Citrus limon and Citrus limetta were collected from Sonapur of Kamrup district and Citrus aurantifolia and Citrus maxima were collected from Pitonipara of Nalbari district of Assam, India.
Sample Preparation
Fresh fruits were peeled off with knife to take the cotyledon. The cotyledon was rinsed with distilled water and dried with absorbent paper. Five g of the fresh seeds were grinded with the help of mortar and pestle and dissolved in water, methanol, hydro methanol, 2-propanol and hydro 2-propanol. The solution was incubated in shaker incubator at 28°C for overnight and following this centrifugation was performed at 10000 rpm for 10 min. The supernatant obtained after centrifugation was used for the further experiments.
 |
Figure 2: Citrus fruits, their size and seeds are presented in the above figure.
|
A. Citrus limon (lemon), b. peeled Citrus limon, c. sliced Citrus limon, d. Citrus limon seeds, e. Citrus limetta (mosumi), f. peeled Citrus limetta, g. sliced Citrus limetta, h. seeds of Citrus limetta, i. Citrus aurantifolia, j. peeled Citrus aurantifolia, k. sliced Citrus aurantifolia, l. seeds of Citrus aurantifolia, m. Citrus maxima (robab), n. peeled Citrus maxima, o. sliced Citrus maxima and p. seeds of Citrus maxima.
Other set of sample was prepared by heating (boiling) at 100°C in doubled distilled water, methanol, hydro-methanol, 2-propanol and hydro 2-propanol in water bath for 2 h. All the prepared samples were stored at -20°C in the ultra-refrigerator for further study.
 |
Figure 3: Samples prepared in water, methanol, hydro-methanol, 2-propanol and hydro 2-propanol from the four different Citrus seeds. a. Citrus limon, b. Citrus limetta, c. Citrus aurantifolia and d. Citrus maxima.
|
Biochemical Analysis
Biochemical analysis of seeds extract was performed for macronutrients viz. total carbohydrate, total soluble protein, total free amino acid, total free fatty acid (acid value) and antioxidant assay.
Estimation of Total Soluble Sugar
Estimation of total soluble sugar was made as per the standard Anthrone method Clegg (1956). To 15 µL seed extract (after centrifugation) 985 µL distilled water was added. Following, 2 mL of Anthrone reagent was added. To the seed extract. The tubes were subjected to mild heating. Resultant color intensity was measured at 630 nm. Quantitative estimation was made from standard curve using glucose as standard.
Estimation of Total Soluble Protein
Total soluble protein was estimated as per the method Lowry et al., (1953). To 50 µL centrifuged seeds extract 950 µL distilled water was added. To this 5 mL solution C was added. This was followed by addition of 0.5 mL Folin-Ciocaltaeu reagent. The tubes were shaken and incubated in dark for half an hour. The developed blue colored solution was measured in a spectrophotometer at 660 nm. Quantitative estimation was made for standard curve using BSA as standard.
Estimation of Free Amino Acid (FAA)
Estimation of free amino acid was made using Ninhydrin reaction by the method of Jayaraman, (1981). 50 µL centrifuged seeds extract were treated with 4.5 mL mild hot 80% ethanol. To this, 3.5 mL Ninhydrin reagent was added. The tubes were warmed in a water bath for about 10 min and absorbance was taken at 570 nm. Estimation was made from standard curve prepared with a mixture of alanine, aspartic acids, tryptophan, proline and lysine in equal proportion.
Estimation of Free Fatty Acid
Free fatty acid was estimated as per the protocol of Cox and Pearson (1962). 1 mL of juice (without centrifugation) was treated with 25 mL of neutral solvent (25 mL diethyl ether, 25 mL of 95% alcohol, 1 mL 1% phenolphthalein solution, the mixture was neutralized with 0.1 N potassium hydroxide). Now, a few drops of phenolphthalein were added and the resultant solution was titrated against 0.001N potassium hydroxide. Free fatty acid was estimated as per the following formula:
Acid value (mg KOH/mL) = [(Titre value × Normality of KOH × 56.1)/Amount of sample in g)]
Estimation of H2O2 Scavenging Activity
H2O2 scavenging activity of seed extracts was determined by using the method developed by Ruch et al., (1989). Different extract concentrations (10 µL and 50 µL/mL) were dissolved in 3.4 mL of 0.1 M phosphate buffer (pH 7.4) and mixed with 600 µL of H2O2 (43 mM). The absorbance of the reaction mixture was taken at 230 nm recorded after 10 min. The H2O2 scavenging activity was calculated by using the following formula:
H2O2 Scavenging activity (%) = [(Ac – As)/Ac] × 100
Where, Ac = control absorbance
As = sample absorbance
Estimation of Total Antioxidant Capacity
The total antioxidant capacity was measured by the method of Pietro et al., (1999). A 50 µL of seeds extracts were taken and the volume was adjusted to 500 µL with distilled water. To this, 4.5 mL phosphomolybdenum reagent (600 mM sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added and mixed properly. Blank containing 500 µL distilled water and 4.5 mL reagent and control with 5mL reagent were prepared. The tubes were capped and kept in boiling water bath at 95 °C for 90 min. After incubation, optical density was taken at 695 nm and 0.25 mg/mL ascorbic acid was used as standard. Total antioxidant capacity was calculated by using the formula:
Total antioxidant capacity (%) = [(As – Ac)/ (Aaa – Ac)] × 100
Where, Ac = control absorbance
As = sample absorbance
Aaa = Ascorbic acid absorbance
Antimicrobial Activity and Determination of MIC of the Extracts by Spectrophotometry Method
The nutrient broth was prepared and from this broth 4.5 mL was added in each of four test tubes labelled as 100%, 75%, 50%, 25% seed extracts concentrations. Five sets of four test tubes containing 4.5 mL nutrient broth were prepared for three test microorganisms. Then, 0.5 mL of each concentration of seeds extracts was added into the respective test tubes. After this step, 0.05 mL test pathogen suspensions were inoculated and inoculated in the respective test tubes. After inoculation, the test tubes were kept in a shaker incubator for overnight at 37 °C and were observed for development of turbidity. O.D. values were recorded at 600 nm. MIC for a given sample considered as the concentration which showed the minimum absorbance, Bansode and Chavan, (2013). It was determined by taking the absorbance and comparing the values at different concentration.
Results
Evaluation of Total Soluble Sugar, Total Soluble Protein, Total Free Amino Acid (FAA) and Free Fatty Acid (FFA) Contents
Total soluble sugar (TSS) exhibited a lot of variation among the seed extracts. In case of seeds before heating, highest total sugar content (72 mg/mL) was found in Citrus maxima hydro 2-propanol whereas lowest (4 mg/mL) was found in the Citrus limetta water. For seeds after heating, the highest total sugar was found in Citrus limon hydro-methanol, Citrus limetta hydro-methanol, Citrus maxima hydro-methanol in 50 mg/mL. Lowest level of total sugar (3 mg/mL) was found in the Citrus limetta water.
Compared to TSS, soluble protein levels are comparatively similar. However, there was significant variation among the different species. In case of seeds before heating, the highest amount of soluble protein (82 mg/mL) was found in the Citrus limon hydro-propanol and lowest level of soluble protein was found (4 mg/mL) in the Citrus maxima water and Citrus maxima methanol. In case of seed after heating, the highest amount of soluble protein (88 mg/mL) was found in the Citrus limon 2-propanol seed extract. Lowest level of soluble protein was found in Citrus limetta methanol with 5 mg/mL.
Highest amount of FAA in case of seeds before heating was recorded in Citrus aurantifolia water with a value of 153 µg/mL and the lowest was recorded in Citrus aurantifolia 2-propanol and Citrus limon 2-propanol with a value of 3 µg/mL. As in case of seeds after heating, the highest amount was recorded in Citrus aurantifolia water with a value of 137 µg/mL and the lowest amount was observed in Citrus limon methanol with a value of 2 µg/mL.
Free fatty acid content is expressed as oleic acid equivalent and like acid number free fatty acid content has been found to be highly variable with similar trend to that of acid number. In case of seeds before heating, the highest free fatty acid content was recorded in Citrus aurantifolia water with a value of 32.5 µg/mL. Lowest free fatty acids were recorded in Citrus limetta water with 9.2 µg/mL; where as in case of heated sample, the highest free fatty acid content was recorded in Citrus aurantifolia water with a value of 24.1 µg/mL and the lowest free fatty acids were recorded in Citrus limetta 2-propanol and Citrus limon 2-propanol with a value of 16.3 µg/mL.
Table 1: TSP, TSS, FAA and FFA of different citrus seed extracts before heating and after heating at 50 µL/mL concentration. All values are expressed in mean ± SEM. Samples taken in triplicate (n=3).
| Sample | TSS (mg/mL) | TSP (mg/mL) | FAA (µg/mL) | FFA (µg/mL) | ||||
| Before heating | After heating | Before heating | After heating | Before heating | After heating | Before heating | After heating | |
| Citrus aurantifolia methanol | 42.0±0.25 | 34.0±0.26 | 6.0±0.24 | 8.0±0.05 | 16.0±0.11 | 4.0±0.10 | 21.9±0.10 | 19.6±0.6 |
| Citrus aurantifolia hydro-methanol | 52.0±0.26 | 46.0±0.33 | 6.0±0.08 | 10.0±0.31 | 30.0 ±0.06 | 5.0±0.11 | 14.6±0.03 | 20.2±0.5 |
| Citrus aurantifolia 2-propanol | 16.0±0.26 | 17.0±0.12 | 68.0±0.3 | 74.0±0.11 | 3.0±0.09 | 21.0±0.11 | 14.6±0.17 | 16.8±0.40 |
| Citrus aurantifolia hydro-propanol | 55.0±0.06 | 48.0±0.25 | 12.0±0.3 | 14.0±0.26 | 40.0±0.08 | 13.0±0.07 | 14.6±0.03 | 16.8±0.17 |
| Citrus limon methanol | 33.0±0.05 | 20.0±0.32 | 6.0±0.15 | 6.0±0.08 | 45.0± 0.09 | 2.0±0.10 | 14.0±0.10 | 19.1±0.30 |
| Citrus limon hydro-methanol | 56.0±0.11 | 50.0±0.30 | 10.0±0.33 | 10.0±0.44 | 23.0± 0.11 | 40.0±0.15 | 14.0±0.03 | 17.4±0.28 |
| Citrus limon 2-propanol | 14.0±0.11 | 10.0±0.05 | 82.0±0.31 | 88.0±0.29 | 3.0±0.15 | 4.0±0.15 | 19.6±0.09 | 16.3±0.19 |
| Citrus limon hydro propanol | 44.0±0.31 | 44.0±0.06 | 8.0±0.33 | 12.0±0.44 | 62.0±0.18 | 24.0±0.10 | 22.4±0.10 | 17.4±0.16 |
| Citrus limetta methanol | 38.0±0.05 | 41.0±0.15 | 7.0±0.30 | 5.0±0.46 | 33.0±0.12 | 17.0±0.14 | 19.6±0.14 | 18.0±0.30 |
| Citrus limetta hydro-methanol | 60.0±0.08 | 50.0±0.15 | 10.0±0.05 | 6.0±0.12 | 25.0±0.17 | 28.0±0.09 | 22.4±0.12 | 17.4±0.19 |
| Citrus limetta 2-propanol | 18.0±0.30 | 17.0±0.30 | 64.0±0.37 | 70.0±0.18 | 8.0±0.09 | 21.0±0.10 | 19.0±0.02 | 16.3±0.18 |
| Citrus limetta hydro-propanol | 46.0±0.15 | 38.0±0.14 | 24.0±0.46 | 18.0±0.08 | 53.0±0.07 | 20.0±0.09 | 15.1±0.05 | 17.4±0.24 |
| Citrus maxima methanol | 28.0±0.08 | 24.0±0.30 | 4.0±0.31 | 6.0±0.39 | 12.0±0.14 | 10.0±0.09 | 29.2±0.08 | 23.0±0.01 |
| Citrus maxima hydro-methanol | 56.0±0.26 | 50.0±0.28 | 12.0±0.23 | 12.0±0.54 | 47.0±0.15 | 24.0±0.18 | 23.0±0.02 | 20.2±0.08 |
| Citrus maxima 2-propanol | 20.0±0.12 | 15.0±0.08 | 76.0±0.28 | 82.0±0.33 | 4.0±0.24 | 21.0±0.12 | 17.4±0.11 | 16.8±0.33 |
| Citrus maxima hydro-propanol | 72.0±0.26 | 36.0±0.27 | 12.0±0.31 | 10.0±0.31 | 50.0±0.14 | 20.0±0.11 | 14.1±0.09 | 18.0±0.32 |
H2O2 Scavenging Activity and Evaluation of Total Antioxidant Capacity
H2O2 was determined to evaluate the total scavenging activity of the different Citrus seed extracts. Among the different seed extracts in case of seeds before heating, the highest activity content was recorded in Citrus aurantifolia propanol with a value 59%. Lowest activity was recorded in Citrus limetta methanol with a value of 12%; whereas in case of heated sample, the highest activity content was recorded in Citrus limon methanol with a value of 67%.
TAC was determined to evaluate the total antioxidant activity of the different Citrus seed extracts. Among the different seed extracts in case of seeds before heating, the highest activity content before heating was recorded in Citrus maxima hydro propanol with a value of 92.5%. Lowest activity was recorded in Citrus limon water 5%; whereas in case of heated sample, the highest activity content after heating was recorded in Citrus aurantifolia 2-propanol with a value of 65%. Lowest activity was recorded in Citrus limetta water with a value of 16.6%.
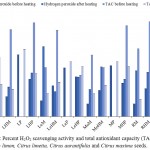 |
Figure 4: Percent H2O2 scavenging activity and total antioxidant capacity (TAC) of Citrus limon, Citrus limetta, Citrus aurantifolia and Citrus maxima seeds.
|
All values are expressed in mean ± SEM and samples were taken in triplicate (n=3). L-Citrus aurantifolia, M-methanol, HM-hydro methanol, P-propanol, HP-hydro propanol, Le- Citrus limon, Mo- Citrus limetta, R- Citrus maxima.
Selection of Microbial Cultures
Microbial culture including Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa were collected from the microbiology laboratory of University of Science and Technology Meghalaya, Baridua – 793101, Meghalaya, India. Microbes were sub-cultured in nutrient broth (pH 7.4) and stored in refrigerator 4°C for further study.
Antimicrobial Activity and MIC Determination of the Extracts
MIC was determined in the form of turbidity and taking optical density (O.D.) at 600 nm in an UV-Vis spectrophotometer. MIC of the different seed extracts was observed for 100% (v/v), 75% (v/v), 50% (v/v) and 25% (v/v) against three test microbes viz. Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli. For Bacillus subtilis, the MIC was found to be at 100%, 100%, 75%, and 75% to the extract of Citrus aurantifolia hydro 2-propanol, Citrus limon methanol, Citrus limetta hydro 2 propanol and Citrus maxima hydro 2-propanol, respectively. For E. coli, the MIC was found to be at 100%, 75%, 100% and 100% for Citrus aurantifolia hydro 2-propanol, Citrus limon hydro 2-propanol, Citrus maxima hydro 2-propanol and Citrus limetta hydro 2 propanol, respectively. For Pseudomonas aeruginosa, the MIC was found to be at 100%, 100%, 75% and 50% for Citrus aurantifolia methanol, Citrus limon methanol, Citrus maxima hydro 2-propanol and Citrus limetta hydro 2 propanol, respectively.
Table 2: Evaluation of antimicrobial activity of different juice extracts on E. coli, Bacillus subtilis and Pseudomonas aeruginosa determination of MIC with respect to extract solution.
| Microbe | Sample | Solvents | Concentration (%) | O.D. (600 nm) |
| Bacillus subtilis | Citrus aurantifolia | Water | ||
| Methanol | 100 | 0.005 | ||
| Hydro methanol | 75 | 0.375 | ||
| 2-propanol | 100 | 0.620 | ||
| Hydro 2-propanol | 100 | 0.002 | ||
| Citrus limon | Water | |||
| Methanol | 100 | 0.009 | ||
| Hydro methanol | 75 | 0.340 | ||
| 2-propanol | 75 | 0.359 | ||
| Hydro 2-propanol | 100 | 0.023 | ||
| Citrus limetta | Water | |||
| Methanol | 100 | 0.022 | ||
| Hydro methanol | 100 | 0.279 | ||
| 2-propanol | 100 | 0.437 | ||
| Hydro 2-propanol | 75 | 0.016 | ||
| Citrus maxima | Water | 100 | 0.287 | |
| Methanol | 100 | 0.138 | ||
| Hydro methanol | 100 | 0.429 | ||
| 2-propanol | 100 | 0.274 | ||
| Hydro 2-propanol | 75 | 0.020 | ||
| Escherichia coli | Citrus aurantifolia | Water | 25 | 0.933 |
| Methanol | 25 | 0.046 | ||
| Hydro methanol | 100 | 0.298 | ||
| 2-propanol | 75 | 0.633 | ||
| Hydro 2-propanol | 100 | 0.024 | ||
| Citrus limon | Water | 75 | 1.495 | |
| Methanol | 50 | 0.029 | ||
| Hydro methanol | 100 | 0.337 | ||
| 2-propanol | 75 | 0.650 | ||
| Hydro 2-propanol | 75 | 0.010 | ||
| Citrus maxima | Water | 100 | 1.408 | |
| Methanol | 100 | 0.041 | ||
| Hydro methanol | 100 | 0.531 | ||
| 2-propanol | 100 | 0.594 | ||
| Hydro 2-propanol | 100 | 0.030 | ||
| Citrus limetta | Water | 100 | 0.364 | |
| Methanol | 100 | 0.106 | ||
| Hydro methanol | 100 | 0.337 | ||
| 2-propanol | 75 | 0.503 | ||
| Hydro 2-propanol | 100 | 0.034 | ||
| Pseudomonas aeroginosa | Citrus aurantifolia | Water | 100 | 0.037 |
| Methanol | 100 | 0.015 | ||
| Hydro methanol | 100 | 0.219 | ||
| 2-propanol | 75 | 0.214 | ||
| Hydro 2-propanol | 50 | 0.620 | ||
| Citrus limon | Water | 25 | 0.343 | |
| Methanol | 100 | 0.021 | ||
| Hydro methanol | 100 | 0.224 | ||
| 2-propanol | 100 | 0.442 | ||
| Hydro 2-propanol | 50 | 0.032 | ||
| Citrus maxima
|
Water | 100 | 0.824 | |
| Methanol | 100 | 0.011 | ||
| Hydro methanol | 25 | 0.232 | ||
| 2-propanol | 100 | 0.361 | ||
| Hydro 2-propanol | 75 | 0.010 | ||
| Citrus limetta | Water | 75 | 0.371 | |
| Methanol | 100 | 0.120 | ||
| Hydro methanol | 100 | 0.323 | ||
| 2-propanol | 75 | 0.608 | ||
| Hydro 2-propanol | 50 | 0.011 |
Discussion
A very least work has been done on nutritional, antioxidant and antimicrobial property of Citrus seed extracts. However, work has been found on the antioxidant and antimicrobial property of Citrus juice Kumari and Handique (2013). They found that the total soluble sugar, total soluble protein, free amino acid and free fatty acid in Citrus reticulata are 102.6 mg/mL, 8.9 mg/mL, 0.50 µg/mL and 0.07 µg/mL. Similarly, the present study revealed that the total soluble sugar (TSS), total soluble protein (TSP), free amino acid (FAA) and free fatty acid (FFA) was for the Citrus seeds extracts are explained in the following. The highest total sugar was observed in Citrus maxima hydro 2-propanol with 72 mg/mL and lowest level of total sugar was found in the Citrus limetta water with 4 mg/mL before heating, after heating the seed extract, the highest total sugar (50 mg/mL) was observed in Citrus limon hydro-methanol, Citrus limetta hydro-methanol, Citrus maxima hydro-methanol and lowest level of total sugar was found in the Citrus limetta water (3 mg/mL). The highest amount of soluble protein was found in the Citrus limon hydro-propanol with 82 mg/mL and lowest level of soluble protein was found the Citrus maxima water and Citrus maxima methanol with 4 mg/mL in the seed extract before heating. In case of heated seed extracts, the highest amount of soluble protein (88 mg/mL) was found in the Citrus limon 2-propanol and lowest level of soluble protein (5 mg/mL) was found in Citrus limetta methanol. For FAA, highest amount of FAA before heating was recorded in Citrus aurantifolia water with a value of 153 mg/mL and the lowest was recorded in Citrus aurantifolia 2-propanol and Citrus limon 2-propanol with a value of 3 mg/mL in case of cool sample. As in case of heated seed extracts, the highest amount was recorded in Citrus aurantifolia water with a value of 137 mg/mL and the lowest amount was observed in Citrus limon methanol with a value of 2 mg/mL. For FFA, the highest free fatty acid content was recorded in Citrus aurantifolia water with a value of 32.5 mg/g and lowest free fatty acids were recorded in Citrus limetta water with 9.2 mg/g in case of cool; whereas in case of heated sample, the highest free fatty acid content was recorded in Citrus aurantifolia water with a value of 24.1 mg/g and the lowest free fatty acids were recorded in Citrus limetta 2-propanol and Citrus limon 2-propanol with a value of 16.3 mg/g.
The antioxidant property of Citrus maxima and the study material used in their work was mainly leaves Kundusen et al., (2011) or juice Oyedepo, (2012). In the present study, for in vitro antioxidant analysis non-enzymatic assay was followed. In non-enzymatic assay in vitro antioxidant activity of the extracts was evaluated by total Antioxidant Capacity (TAC) and H2O2 scavenging activity. For TAC, the highest activity content was recorded in Citrus aurantifolia 2-propanol with a value of 59.2% and lowest activity was recorded in Citrus limon water 5% in case of sample before heating; whereas in heated sample, the highest activity content was recorded in Citrus aurantifolia 2-propanol with a value of 64.8% and lowest activity was recorded in Citrus limetta water with a value of 8%. For H2O2 scavenging activity, the highest activity content was recorded in Citrus maxima hydro-propanol with a value 92.5% and lowest activity was recorded in Citrus limon hydro-propanol with a value of 39.6% in case of cooled sample; whereas in case of heated sample, the highest activity content was recorded in Citrus limon methanol with a value of 67.2 % and lowest activity was recorded in Citrus limon hydro-methanol with a value of 23.9% (Table 1).
Works of; Tao et al., (2008) and Mathur et al., (2011) proved the antimicrobial potential of different parts of Citrus maxima like leaves, fruit wastes and essential oil against microbes like Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus, etc. In our present study, antimicrobial activity of the Citrus seed extracts was studied against Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa by spectrophotometry method. MIC was observed in the form of turbidity and taking the optical density (OD) at 600 nm. In this method, MIC of seed extracts against Bacillus subtilis was found in Citrus aurantifolia hydro 2-propanol 100%, Citrus limon methanol seed extract (100%), Citrus limetta hydro 2-propanol (75%) and Citrus maxima hydro 2-propanol (75%). The MIC towards E. coli was found in Citrus aurantifolia hydro 2-propanol (75%), Citrus limon hydro 2-propanol (75%), Citrus maxima hydro 2-propanol (100%) and Citrus limetta hydro 2-propanol (100%). The MIC of Citrus aurantifolia methanol, Citrus limon methanol, Citrus maxima hydro 2-propanol and Citrus limetta hydro 2-propanol to Pseudomonas aeroginosa was 100%, 100%, 75% and 50%, respectively (Table 2).
The concept of eating Citrus fruits in the form of raw, juice and powder has been tremendously increasing in the community as because people believe that the Citrus fruits contain antioxidative compounds, antimicrobial properties, etc Gillman et al., (1995); Rimm et al., (1996); Kumari and Ahad (2017). Eating Citrus fruits as a medicine to recover scurvy is an old practice done by the common people from few decades, Rapisarada et al., (1999). The waste seeds of the Citrus fruits carried various antioxidative, nutritive value and antimicrobial property that was proved in this study. The seed extracts of the Citrus limon, Citrus maxima, Citrus aurantifolia and Citrus limetta contained tremendous amount of soluble free sugars, soluble proteins, free amino acids and free fatty acids. They also possessed high H2O2 scavenging activity, antioxidative capacities as well as antimicrobial properties. ROS after crossing the threshold limit in any organism is harmful and damage lipid membranes and degrade proteins and amino acids, Tchimene et al., (2016). Eating Citrus fruits in raw form, juices can reduce the oxidative stress as the juice contains non enzymatic antioxidants such as phenolics, flavonoids and ascorbic acid, tocopherol, vitamiins, etc. Kumari et al., (2016). We also looked into the effects of cooking temperature on the biochemical parameters (free sugars, amino acids, fatty acids, etc.), antioxidative capacities as well as antimicrobial properties. The results determined that heat also slightly reduce these properties. Therefore, eating raw seed extracts, or powdered form of seeds is more effective against oxidative stress and microbial infections.
Conclusion
The Citrus species namely Citrus limon, Citrus limetta, Citrus maxima and Citrus aurantifolia are predominantly found in the North-Eastern region of India. These fruits are rich in biomolecules (free sugars, amino acids, fatty acids) and carry antioxidant and antimicrobial properties. In general, the seeds of these fruits are thrown/ disposed after eating the fruits. In this study, we have looked into the free sugars, amino acids and fatty acids contents of Citrus limon, Citrus limetta, Citrus maxima and Citrus aurantifolia seeds and their antioxidative capacities and antimicrobial properties before and after heating. Among the seeds of the Citrus, Citrus limon seeds carried the highest antioxidative capacities and antimicrobial properties before and after heating. All the Citrus seeds carried antioxidative capacities and antimicrobial properties and hence these seeds can be processed into pharmaceutical products to treat against ROS stresses and different antimicrobial diseases. The suggestion is to eat the seed as raw or in the powdered form to achieve the maximum response against oxidative stresses and microbial infections. The product will be a cheaper one and can attain higher market value and popularity as this product do not carry any side effects. The source (seed) is considered as waste. So, the waste can be converted into useful pharmaceutical product. This will increase the economic status of N. E. India.
Acknowledgements
The authors acknowledge University of Science and Technology, Meghalaya, Baridua – 793101, Ri-Bhoi for providing all the facilities.
Conflict of Interest
There is no conflict of interest.
References
- Al-Jedah J. H., Robinson R. K. Nutritive Value and Microbiological Safety of Fresh Fruit Juices sold through Retail Qutlets in Qatar. Parkistan J. Nutri. 2002;1(2):79-81.
- Al-Shuneigat J., Cox S. D., Markham J. L. Effects of a topical essential oil containing formulation on biofilm-forming coagulase-negative staphylococci. Lett. Appl. Microbiol. 2005; 41: 52-55.
- Bansode D. S., Chavan M. D. Evaluation of antimicrobial activity and phytochemical analysis of papaya and pineapple fruit juices against selected enteric pathogens. Int. J. Pharm. Bio. Sci., 2013;4(2):1176-1184.
- Borthakur D. N. Agriculture of North Eastern region. Bee cee Prakasshan, Guwahati, India. 1992.
- Caccioni D. R. L., Guizzardi M., Biondi D. M., Renda A., Ruberto G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicilliumn digitatum and Penicillium italicum . Int. J. Food Microbiology. 1998; 43:73-79.
- Clegg K. M. The application of Anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agri. 1995;70:409-44.
- Cox H. E., Pearson D. The chemical analysis of foods, Chemical Publishing Co. Inc. New York; 1962; 420.
- El-Adawy T. A., El-Bedawy A. A., Rahma E. H., Gafar A. M. Properties of some citrus seeds. Evaluation as a new source of protein and oil.Food / Nahrung. 1999;43(6):385-391.
- Gillman M. W., Cupples L. A., Posner B. M., Ellison R. C., Castelli W. P., Wolf P. A. Protective effect of fruits and vegetables on development of stroke in men. J. Med. Assoc. 1995;273:1113 –1117.
- Gislene G. F., Locatelli N. J., Paulo C. F., Giuliana L. S. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Brazilian. J. Microbiol. 2000; 31:247-256.
- Hammer K. A., Carson C. F., Riley T. V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985-990.
- Hayes and Markovic. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 2002;40(4):535-43.
- Jayaraman J. Laboratory Manual of biochemistry. Wiley Eastern ltd. New Delhi. 1981.
- Kumari S., Ahad R. I. A. In vitro antioxidant, antimicrobial properties and total phenolic contents of Citrus limetta collected from Sonapur, Assam, India. Int. J. Herbal Med. 2017;5(5):89-93.
- Kumari S., Handique A. K. Total Phenolic content and in vitro antioxidant activity of four indigenous Citrus fruits of North East India. Int. J. Sci.Eng. Res. 2013;4(6):2125.
- Kumari S., Siddique F. M. H, Gupta S. D., Baruah A. Antimicrobial property of different parts of Citrus limon. IOSR J. Biotechnol. Biochem. 2016;2(2): 53-55.
- Kundusen S., Gupta M., Mazumder M. K., Haldar P. K., Saha P., Bala P. Antitumor Activity of Citrus maxima (Burm.) Merr. Leaves in Ehrlich’s Ascites Carcinoma Cell-Treated Mice. ISRN Pharmacology. 2011. doi:10.5402/2011/13873.
- Lowry O. H., Rosenbrough N. J., Furr A. L., Randall R. J. Protein measurement with folin-phenol reagent. J. Biol. Chem.1953; 193: 267-275.
- Mathur A., Verma S., Purohit R, Gupta V., Prasad V. K., Mathur D., Singh S. K. Evalution of in vitro antimicrobial & antioxidant activity. J. Biotechnol. Biotherapeu. 2011; 2229-2278.
- Oyedepo T. A. Antioxidant potential of Citrus maxima fruit juice in rat. Global Adv. Res. J. Med. Med. Sci. 2012;1(5):122-126.
- Pietro P., Pineda M., Anguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal Biochem. 1999;269:337-341.
- Pultrini A. M., Galindo L. A., Costa M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. 2006;78:15.
- Rapisararda P. T., Antonio I. C., Rossella B., Prancesco O. P., Annaand A. Antioxidant effectiveness as influenced by phenolic content of fresh orange juice. J. Agric. Food Chem., 1999; 47: 4718-4723.
- Reda S. Y., Leal E. S., Batista E. A. C. Characterization of Rangpur lime (Citrus limonia Osbeck) and “sicilian” lemon (Citrus limon) seed oils, an agro-industrial waste. Cienciae Technol. Alimentos. 2005;25(4):672–676.
- Rimm E. B., Aschiero A., Giovannucci E., Spiegelman D., Stamper M. J., Willett W. C. Vegetable, fruits and cereal fiber intake and risk of coronary heart diseases among men. J. American Med. Assoc. 1996;275: 447–451.
- Ruch R. J., Cheng S. J., Klaunig J. E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989; (10):1003.
- Saıdani M., Dhifi W., Marzouk B. Lipid evaluation of some Tunisian Citrus seeds. J. Food Lipids. 11(3);242–250.
- Seenivasan P., Manickkam J. Savarimuthu I. In vitroantibacterial activity of some plant essential oils. BMC Complem. Altern. M., 2006;6:39.
- Shoji T., Masumoto S., Moriichi N., Kobori M., Kanda T., Shinmoto H. Pocyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J. Agric. Food Chem. 2005;53:6105-6111.
- Tao N-Guo, Liu Y-J., Zhang J. H., Zeng H. Y., Tang Y. F., Zhang M. Chemical composition of essential oil from the peel of Satsuma mandarin. Afr. J. Biotechnol. 2008;7:1261-1264.
- Tchimene M. K., Nwaehujor C. O., Ezenwali M., Okoli C. C., Iwu M. M. Free radical Scavenging aactivity of Lupeol isolated from the methanol leaf extract of Crateva adansonii Oliv. (Capparidaceae). Int. J. Pharmacog. Phytochem. Res. 2016; 8(3):419-426.
- Tepe B. D, Daferera S. M., Polissiou M., Sokmen A. In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii. J. Agric. Food Chem. 2004;52: 1132-1137.
- Zhang Q. A., Zhang Z., Yue X., Fan X., Li T., Chen S. Response surface optimization of ultrasound-assisted oil extraction from autoclaved almond powder. Food Chemistry. 2009;116(2): 513–518.

This work is licensed under a Creative Commons Attribution 4.0 International License.





