Manuscript accepted on : 27 February 2012
Published online on: --
Bilal Ahmad Bhat1*, Imtiyaz Ahmad Bhat1, Santosh Vishwakarma1, Alok Verma2 and Geeta Saxena1
1Department of Zoology, Govt. Science and Commerce College Benazir, Bhopal - 462 008, India. 2Department of Zoology, Govt. College Lateri vidisha - 464 114, India.
ABSTRACT: Dichlorvos, a broad spectrum Organophosphate insecticide is a potential toxic pollutant, adversely affecting the fauna of aquatic ecosystem. The objective of the present work was to evaluate the acute toxicity and behavioral responses of Dichlorvos on Labeo rohita under static conditions. The median lethal concentration of the insecticide was found to be 16.71ppm and one tenth of LC50 (1.67ppm) was studied for sub acute studies. Data obtained from the Dichlorvos acute toxicity tests were evaluated using Finney's Probit Analysis statistical method. Behavioral patterns were studied in lethal (1, 2, 3 & 4 d) and sublethal concentration (1, 5, 10 & 15 d). The test fish showed erratic swimming, increased surfacing, copious mucus secretion, loss of equilibrium and hitting to the walls of test tank before finally sinking to the bottom just before death. Fish under sublethal concentration were found under stress, but that was not fatal.
KEYWORDS: Acute toxicity; Dichlorvos, LC50; Labeo rohita; Behavioral changes
Download this article as:| Copy the following to cite this article: Bhat B. A, Bhat I. A, Vishwakarma S, Verma A, Saxena G. Acute Toxicity and Behavioral Responses of Labeo Rohita (Hamilton) to an Organophosphate (Dichlorvos). Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Bhat B. A, Bhat I. A, Vishwakarma S, Verma A, Saxena G. Acute Toxicity and Behavioral Responses of Labeo Rohita (Hamilton) to an Organophosphate (Dichlorvos). Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9750 |
Introduction
Aquatic ecosystems that run through agricultural areas have high probability of being contaminated by runoff and ground water leaching by variety of pesticides. Among different classes of pesticides, organophosphate pesticides are finding increasing use in recent years since they are biodegradable and, therefore, persist in the environment only for a short time. Because of their low persistence, repeated applications of these pesticides are being practiced for the control of pests in agricultural fields and thereby large quantities find their way into water bodies (Jyothi and Narayan 1999). Dichlorvos is recommended for application as a high or a low volume spray on crops such as paddy, wheat, soyabean, apple, sugarcane, mustard, sunflower and cashew. The Environment Protection Agency (EPA) has classified dichlorvos as toxicity class 1 highly toxic (URL: 1).
Behavior is considered as promising tool in Ecotoxicology. (Drummond and Russom, 1999; Cohn and Macphail, 1996). The assessment of the ecotoxicological risks caused by pesticides to ecosystems is based on data on toxicity and effect of pesticide preparations to non target organisms. Fishes are among the group of non target aquatic organisms. Since fishes are the best indicator to check the level of pollution in aquatic ecosystem. Hence the present study was undertaken to evaluate aquatic toxicity of dichlorvos with special emphasis on behavioral changes of freshwater teleost Labeo rohita exposed to lethal and sublethal concentration of technical grade Dichlorvos.
Material and Methods
Healthy and active adult Labeo rohita were obtained from Patra and Bhadbhade fish farms barkhedi and bhadbhada Bhopal M.P respectively. They weighed 55g±1g and their length was in the range 15cm±1. They were brought to laboratory carefully in oxygen filled polythene bags in card board boxes to avoid any injury and disinfected by giving a bath for five minutes in KMno4 solution. Thereafter, they were transferred to glass aquariums filled with dechlorinated water. The fishes were acclimated to the laboratory conditions for at least 20 days prior to the experiment. During acclimatization fishes were fed daily with commercial fish food which was given at morning hours. Water was replaced every 24h after feeding in order to maintain a healthy environment for the fish during acclimation and experimental period. This ensures sufficient oxygen supply for the fish and the environment is devoid of any accumulated metabolic wastes. Dead fishes when ever located were removed immediately to avoid fouling of the water.
Water quality characteristics were determined and maintained. Nuvan (dichlorvos 76% EC) manufactured by Syngenta India ltd. 14, J. Tata road, Mumbai was used for evaluation of its toxicity to fish. For determining LC50 concentration different stock solutions were prepared, separate glass aquariums were taken and different concentrations of Dichlorvos were added from the stock solution. Simultaneously a control set was run with the experiment. During assay no food was administered to fishes. The LC50 concentration for 96h was calculated by probit analysis method of Finney’s (1971). The control and Dichlorvos exposed fish were kept under continuous observation during experimental periods.
Results
The acute toxicity of Dichlorvos for the freshwater fish Labeo rohita was found to be 16.71ppm. The maximum concentration at which zero percent mortality and minimum concentration at which 100% mortality of Labeo rohita were observed at 14 & 19ppm respectively. The LC50 concentration for 96h was calculated by probit analysis method of Finney’s (1971). Table 1 shows the relation between the Dichlorvos concentration and the mortality rate of Labeo rohita and the graph below shows the plot of Finney’s probits against log10 conc. for calculating LC50 value.
Table 1
| Conc.(mg/L) | Log10Conc. | Total No. | No. Dead | %Mortality | Probit.
|
|
14 |
1.1461 |
10 |
0 |
0% |
– |
|
15 |
1.1761 |
10 |
1 |
10% |
3.72 |
|
16 |
1.2041 |
10 |
3 |
30% |
4.48 |
|
17 |
1.2304 |
10 |
6 |
60% |
5.25 |
|
18 |
1.2553 |
10 |
8 |
80% |
5.84 |
|
19 |
1.2788 |
10 |
10 |
100 % |
– |
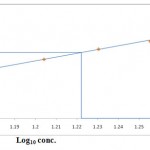 |
Figure 1
|
Log10 conc.
Behavioral changes are physiological responses shown by the animal, which are often used as the sensitive measure of stress syndrome in the organisms expecting it, consequently the behavioral changes were observed in control and exposed fishes. The test fish exhibited irregular, erratic and darting swimming movements and loss of equilibrium. They slowly became lethargic, hyper excited, restless and secreted excess mucus all over their bodies. The fish exhibited peculiar behavior of trying to leap out from the pesticide medium which can be viewed as an escaping phenomenon. The frequency of surfacing was greater on the second day. Partially extended fins and wide opening of the mouth and opercular coverings followed by hyperextension of all fins were found and on third day fish was in a state of excitement. On the fourth day they lost their equilibrium and response to external stimuli. They often spiral rolled at intervals and finally the fishes sank to bottom with their least operculum movements and died with their mouth opened.
In sublethal treatment, the schooling behavior of the fish was slowly disrupted during the first day. The hyperactivity, excitement, hyperventilation etc was not influenced on exposure to the sublethal concentration of the Dichlorvos at 5 and 10 days. Further fishes at 15 days of exposure exhibited free and normal swimming.
Discussion
Fish mortality due to pesticide exposure mainly depends upon its sensitivity to the toxicant, its concentration and duration of exposure. The LC50 values of Dichlorvos for different fishes has been reported by various workers, as in Cyprinus carpio 6gm it was 0.34ppm for 96h (Verma et al., 1981), in Clarias batrachus 31gm it was 8.9ppm for 96h (Verma et al.,1983), in Gambusia affinis .3gm it was 5.3ppm for 96h (Johnson and Finley (1980), in Grey Mullet Liza parisa 6.50gm it was 0.482ppm for 96h (Mahopatra et al., 1992), in Cirrhinus mrigala it was 9.1ppm for 96h (Velmurugan et al., 2009) and in Ctenopharyngodon idella it was 13.1ppm for 24h (K.S Tilak and Swarna Kumari 2009). In the present study the LC50 value of Dichlorvos for 96h in Labeo rohita was found to be 16.71ppm (size 15cm, wt. 55gm). It is clear from earlier studies that LC50 of pesticides for a freshwater fish varies from species to species and in the same species under the influence of number of factors including size and time of exposure.
In the present study, the control fish behaved in natural manner, but in the toxic environment, fishes exhibited a number of abnormalities in their behavior. Within a few minutes of exposure to higher concentrations, the fishes exhibited irregular, erratic and darting swimming movements and swimming at the surface of the water. This surfacing phenomenon was more in fish exposed to lethal concentration and sublethal concentration over the control fish. Hyper excitation, flaring of gills, increase in production of mucus, hitting against the walls of container resulting in oozing of blood from the snout of fishes and loss of equilibrium which is due to inhibition of AchE activity leading to the accumulation of acetylcholine in cholinergic synapses ending up with hyperstimulation (Mushigeri and David, 2005). They slowly become lethargic, hyper excited, restless and secreted excess mucus all over the body. Mucus secretion in fish forms a barrier between body and toxic media thereby probably reduces contact of toxicant so as to minimize its effect or to eliminate it through epidermal mucus. Similar observations were made by Rao et al., (2003) and Parma de croux et al., (2002). Gulping of air at the surface, swimming at the water surface, disrupted schooling behavior was seen on the first day itself in lethal exposure period, which is in accordance with the observations made by Ural & Simsek (2006). Partially extended fins and single wide opening of the mouth and opercular covering accompanied by hyperextension of all fins were found on the third day.
Defecation is considerably increased in the exposed fishes in comparison to control group. This is in accordance with typical organophosphate toxicity involving hyper stimulation of muscarinic receptors in the smooth muscles of the end organs viz, gastrointestinal tract and secretory glands. On the fourth day they lost their equilibrium and response to external stimuli. The fish eventually died with their mouth and operculum wide opened (Bonita, L.B., 2004)
In sublethal exposure fish body lean towards abdomen position compared to control fish and was found under stress, but was not fatal. Leaning of fish indicate reduced amount of dietary protein consumed by the fish at pesticide stress, which was immediately utilized and was not stored in the body weight (Kalavathy et al., 2001). In sublethal treatment, the schooling behavior of fish was slowly disturbed during the first day itself. The ventilation rate was increased, but hyperactivity, excitement, hyperventilation etc. were not influenced on exposure to the sublethal concentration of Dichlorvos on day Ist and five.
In the present study the abnormal changes in the fish exposed to lethal concentration of Nuvan are time dependent. However, the normal behavior of the fish at 10 and 15 days on exposure to sublethal concentrations indicates its adaptability to the sublethal concentration due to long term exposure of Dichlorvos. The analysis of data from the present investigation evidenced that Dichlorvos is toxic and had profound impact on behavior in Labeo rohita in both lethal and sublethal concentrations. Hence this type of study can be useful to compare the sensitivity of the various species of aquatic animals and potency of chemicals using LC50 values and to derive safe environmental concentration by which there is not lethality and stress to the animals especially to the non target organisms. Further studies on toxicity of Dichlorvos and its combination with other pesticides on mortality and behavior of fish in the laboratory and field are required.
References
- Jyothi, B., Narayan, G., Certain pesticide induced carbohydrate metabolic disorders in the serum of freshwater fish Clarias batrachus (Linn.). Food. Chem. Toxicol., 37, 417-421 (1999).
- Drummond, R.A. and Russom, C.L., Behavioral toxicity syndromes, a promising tool for assessing toxicity mechanisms in juvenile fathead minnows, Environ. Toxicol. Chem., 9: 37-46 (1990).
- Cohn, J. and MacPhail, R.C., Ethological and experimental approaches to behavior analysis, implications for ecotoxicology. Environ. Health Perspect, 104: 299-304 (1996).
- Finney, D.J., Probit analysis, 3rd (Ed.), Cambridge University Press, London, 333 pp (1971).
- B.C. Mohapatra and A. Noble., Liver and kidney damage in grey mullet Liza parisa on exposure to an Organophosphate Nuvan. j. mar. boil. Ass. India, 34 (1&2): 218-221 (1992).
- Velmurgan, B., Selvanayagam, M., Cenigz, E.I., Unlu, E., Histpathological changes in the gill and liver tissues of freshwaterfish, Cirrhinus mrigala exposed to Dichlorvos. Braz. Arch. Biol. Technol. v.52n. 5:pp. 1291-1296 (2009).
- Tilak, K.S. and R. Swarna Kumari., Toxicity of Nuvan, an organophosphate to freshwater fish Ctenopharyngodon idella and its effect on oxygen consumption. J. of Environmental Biology, 30(6), 1031-1033 (2009).
- Mushigeri, S.B. and David, M., Fenvalerate induced changes in the Ach and associated AChE activity in different tissues of fish, Cirrhinus mrigala (Hamilton) under lethal and sub-lethal exposure period. Environmental Toxicology and Pharmacology, 20 :65 -72 (2005).
- Parma de Croux, M.J., Loteste, A. and Cazenave, J., Inhibition of plasma cholinesterase and acute toxicity of monocrotophos in Neotropical fish, Prochilodus lineatus (Pisces, Curimatidae). Bull. Environ. Contam. Toxicol., 69: 356-362 (2002).
- Ural, M.S. and Simsek Koprucu, S., Acute toxicity of dichlorvos on fingerling European catfish, Silurus glanis. Bull. Environ. Contam. Toxicol., 76: 871-876 (2006).
- Bonita, L.B., Toxicology of the nervous system. in: Hodgson, (Ed) A T.B. of modern toxicology. John Wiley and sons Inc. New Jersey, USA, pp: 279-297 ( 2004).
- Kalavathy, K., Sivakumar, A.A. and Chandran, R., Toxic effects of the pesticide dimethoate on the fish, Sarotherodon mossambicus. J. Ecol. Res. Bio., 2: 27-32 (2001).
- APHA., Standard methods for the examination of water and waste water, American Public Health Association. Washington, DC ( 2005).
(Visited 153 times, 1 visits today)

This work is licensed under a Creative Commons Attribution 4.0 International License.





