Manuscript accepted on : 08 November 2011
Published online on: --
P. Jawahar Babu*, K. V. Rajesh, P. Durga Prasad, K. Manjusha and K. Vindya Rani
Department of Biotechnology, Bapatla Engineering College, Bapatla, Andhra Pradesh - 522 101 India.
Correspondence Author E-mail: jawaharbiotech@gmail.com
ABSTRACT: Acute lymphoblastic leukemia is more common in children, accounting for 85% of childhood leukemias. The incidence of this disease is highest in the three to four year old age group, falling off by ten years. L-asparaginase is an effective antineoplastic agent, used in the acute lymphoblastic leukemia chemotherapy. It has been an integral part of combination chemotherapy protocols of pediatric acute lymphoblastic leukemia for almost 3 decades. It is the first enzyme to be therapeutically effective in human malignant disease. Native or PEGylated L-asparaginase (ASNase or PEG-ASNase) are highly specific for the deamination of L-asparagine (Asn) to aspartic acid and ammonia. Depletion of Laspargine leads to a nutritional deprivation and inhibition of protein biosynthesis, resulting in apoptosis in T-lymphoblastic leukemia’s, which require L-aspargine from external sources. This review article comprises detailed information about L-asparaginase historical developments of therapy, its mechanism of action, Advantages of PEG-Lasparaginase versus native preparations, Pharmacokinetic and Pharmacodynamics models of the enzyme, Clinical potential ,its efficacy and host resistance to ASNase.
KEYWORDS: Acute lymphoblastic leukemia; Asparaginase; Enzymatic activity; PEGylation; Antibodies; Pharmacokinetics; Pharmacodynamics
Download this article as:| Copy the following to cite this article: Babu P. J, Rajesh K. V, Prasad P. D, Manjusha K, Rani K. V. Role of L-Asparaginase as an Effective Chemotherapeutic agent for Acute Lymphoblastic Leukemia in Children’s. Biosci Biotech Res Asia 2011;8(1). |
| Copy the following to cite this URL: Babu P. J, Rajesh K. V, Prasad P. D, Manjusha K, Rani K. V. Role of L-Asparaginase as an Effective Chemotherapeutic agent for Acute Lymphoblastic Leukemia in Children’s. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=21233 |
Introduction
Acute lymphoblastic leukemia is the most common childhood malignancy representing 35% all cancer in American children under the age of 15 years. About 4000 cases of acute lymphoblastic leukemia (ALL) are diagnosed every year in the US and many more throughout the world. The majority of these cases are in children and young adults, making ALL the most common form of malignancy in these age groups. ASNases have been the cornerstone of ALL therapies since the late 1970s. L-Asparaginase (L-asparagine amido hydrolase, EC 3.5.1.1) catalyzes the hydrolytic cleavage of the substrate L-asparagine to form L-aspartate and ammonia. Its distinct antitumor activity is an incredible feature, due to which the enzyme has found wide application in pharmaceutical science as an effective chemotherapeutic agent against the acute lymphoblastic leukemia and non-Hodgkin’s lymphoma. It has been a mainstay of combination chemotherapy protocols used in treatment of pediatric ALL for almost 30 years [1–6]. Based on this, it has also been included in most contemporary, multi-agent regimens for adult ALL [7, 8]. The major limitation to the use of l-asparaginase is dose limiting clinical hypersensitivity, which develops in 3–78% of patients treated with unmodified forms of enzyme [3,9–11].Over the last 10 years, PEG-L-asparaginase as an alternate form of L-asparaginase seems to have redressed the problems being faced with the native preparations [12,13].
History of L-Asparaginase
The history of the enzyme L-Asparaginase dates back when Clementi (1922) [14] reported the presence of this enzyme in the blood of guinea pigs. The identification of the antitumor property of L-Asparaginase began when Kidd (1953) [15, 16] found that the injection of guinea pig serum caused a complete regression of lymphosarcoma in mice and sera from other species were without any effect. This cytolytic activity was not present in horse or rabbit serum. Neuman and McCoy [17] extended these observations in 1956. They demonstrated that the growth of cell line derived from Walker carcinosarcoma required L-asparagine. Haley et al.) [18] in 1961 obtained similar results, using a mouse leukemia cell line. It was Broome in 1961 while working in Kidd’s laboratory, who compared Kidd’s finding of growth inhibition with the earliest observation by Clementi, and succeeded in concluding that the anti lymphoma activity in guinea pig sera was due to L-Asparaginase [19]. Further investigations of the same author confirmed its therapeutic potential [20]. Yellin and Wriston in 1966, succeeded in partial purification of two isoforms of L- Asparaginase from the serum of guinea pig [21]. Interestingly, only one isoform exhibited anti lymphoma activity in vivo [22]. Since the extraction of this enzyme from the guinea pig serum in sufficient amounts was difficult, other sources like microbes were looked into. Mashburn and Wriston, in 1964 and Campbell and Mashburn in 1969 reported the purification of E. coli L-Asparaginase, and demonstrated its tumoricidal activity similar to that of guinea pig sera [23]. These findings provided a practical base for large-scale production of enzyme for pre- clinical and clinical studies [24]. Oettgen et al. [25] in 1967 were first to show the efficacy of L-Asparaginase in humans with leukaemia. Another important contemporary observation was that of Old et al., demonstrating in vivo antitumor activity in a dog model with spontaneous lymphosarcoma. Parenteral therapy with foreign proteins in humans has limitation due to the fundamental problem of the drug’s immunogenicity. Hypersensitivity reactions to the E. coli L-Asparaginase were recognized to be fairly common. A combined study on L-Asparaginases from E. coli and Erwinia caratovora showed the lack of cross-reactivity [26]. Both enzymes, however exhibited high rate of immunogenicity. Attempts were being made to reduce the immunogenicity of the drug while preserving its enzyme activity. Over several decades from the discovery of the enzyme it proved to be important in the cancer therapy either in native or modified forms from varied sources.
Classification of L-Asparaginases
L-ASNases have been isolated and characterized from various microorganisms, including many gram-negative bacteria, mycobacteria, yeasts, and molds, as well as from plants and from the plasma of certain vertebrates [27]. All L-ASNases can be divided into three classes based on amino acid sequence. The first class includes the bacterial type II L-ASNases. [28] .Type II enzymes can also hydrolyze L-Gln. In the cases where L-Gln is the better substrate, the enzymes are termed glutaminase asparaginases (EC 3.5.1.38). Crystallographic studies of glutaminase- asparaginases [29] have revealed that they share the same tertiary and quaternary structure with type II L- ASNases. A second-class of L-ASNases exists in plants, namely the plant-type L-ASNases, with no homology to the bacterial-type enzymes [30]. The asparaginases from plants show about 60% sequence similarity to aspartylglucosaminidases (EC 3.5.1.26). Aspartylglucosaminidases in humans catalyze the hydrolysis of the glycosidic bond between the sugar chains and the L-Asn side-chain, the last stage of degradation of glycosylated proteins [31]. The third class of L-ASNases includes the L-ASNase from Rhizobium etli (ReA), with no homology to other ASNases [32].
Chemistry and Mechanism of Action of L-Asparaginase
Tumor cells require huge amount of asparagine to keep up with their rapid malignant growth. This means they use both asparagine from blood serum as well as what they can make themselves to satisfy their large L-asparagine demand. L-Asparaginase as a drug exploits this unusually high requirement tumor cells have for the amino acid asparagine. L-Asparaginase catalyses the hydrolysis of L-asparagine to L-aspartic acid and ammonia (Fig. 1). L-Asparaginase depletes serum asparagine and kills tumor cells by depriving them of an essential factor required for protein synthesis. Healthy cells however escape unaffected, as they are capable of synthesizing asparagine themselves with the help of the enzyme l-asparagine synthetase, which is present in sufficient amounts. Cell cycle arrest in the G1 phase has been reported in the murine L5178Y cell line and the MOLT-4 human T-lymphoblastoid line, resulting in apoptosis. It has been reported that there is a requirement of a functional p53 protein for L-Asparaginase to produce apoptosis as observed in HL60 promyelocytic leukaemia cell lines [33]. However, there are contradictory results in few other cell lines. Physiological differences between the tumor and normal cells in amino acid production aid in the treatment of cancer with Asparaginase.
Three Dimensional Structure of L-Asparginase
The first reported three dimensional structure of the family was that of Acinetobacter glutaminasificans asparaginaseglutaminase. This model, however, proved useful in molecular replacement trials that led to the crystal structure determination of EcAII at 2.3 Å resolution in 1993 [34]. Subsequently, crystal structures of a number of LASNases were determined, including ErA , Wolinella succinogenes L-ASNase (WsA) , Erwinia carotovora (EwA) and related asparaginase-glutaminases from Acinetobacter glutaminasificans (AgA) and Pseudomonas fluorescence (PgA) . The highest resolution so far is that of ErA at 1.0 Å . Until now, the only reported 3D X-ray structures of type I LASNases are those from the hyperthermophilic archaeon Pyrococcus horikoshii (PhA) and from E. coli (AnsA) [35].
All asparaginases are homotetramers with 222-symmetry and a molecular mass in the range 140–150 kDa, with a highly conserved overall fold [31]. They are composed of four identical subunits denoted A, B, C and D.The enzyme monomer consists of ~330 amino acid residues arranged in two domains: a large N-terminal domain and a smaller C-terminal domain connected by a linker of ~26 residues (Fig. (2)). Both domains are characterised by an α/β fold. The N-terminal domain contains an 8-stranded mixed β-sheet. The smaller C-terminal domain comprises a four-stranded parallel β-sheet and four α-helices. The overall dimensions of the molecule are approximately 48 x 58 x 62 Å. A distinguishing feature of all the L-ASNases is the presence of a left-handed crossover between strands β4 and β5. This type of protein fold is rarely observed in protein structuresbut is typical to L-ASNases [36] and contributes to the activity of the enzyme. The residues involved in the crossover form part of the active site and are evolutionarily conserved. Another characteristic of the L-ASNase structures is the highly conserved Thr198 (EcAII crystal structure numbering) that contributes to the folding of the protein in the correct conformation [34].
Active Site and Reaction Mechanism
Crystallographic data on bacterial L-ASNases have shown a high degree of structural similarity at the active sites and the presence of strictly conserved residues. Four identical non-cooperative active sites have been identified that are formed at the subunit interfaces: two between subunits A and C and two between B and D. The N- and C-terminal domains of the subunits are involved in the formation of each active site, thus the L-ASNase homotetramers are best described as dimers of intimate dimers. The active site is formed by strictly conserved residues. Part of the active site is a flexible loop (between residues 10 and 40) that contains the two important residues Thr-12 and Tyr-25 (Aghaiypour et al., 2002) [37]. This flexible loop exists in different conformations depending on the presence or absence of a bound ligand in the active site. EcAII residues Thr12, Tyr25, Ser58, Gln59, Thr89, Asp90, Ala114 and Lys162 from one subunit, and Asn248 and Glu283 from the other intimate subunit form the binding pocket. Conserved water molecules in the active site play an active role in stabilizing the conformation of the flexible loop and mediating interactions between the substrate and surrounding residues.
The active site is characterized by the presence of two Thr residues (Thr89 and Thr12 in EcAII). Both threonines have been found indispensable for the reaction mechanism A hydrogen bond betweenThr89 hydroxyl group and the amino group of Lys162 was no longer formed in the mutant, suggesting the importance of the latter residue as well. The presence of an Asp residue (Asp90) near Thr89 and Lys162 led to the proposal of a catalytic triad mechanism operated similarly to that of serine proteases. Furthermore, a structural study with diazo analogs of glutamine and asparagine that acted as suicide inhibitors of the enzyme also supported the role of Thr12 as the primary nucleophile [38]. Thus, the question which of these two Thr residues acts as the primary nucleophile has been under continuous discussion. A hydrogen-bond distance between Thr12 and Tyr25 further suggested that Tyr25 could be involved in proton abstraction by enhancing the nucleophilicity of Thr12. Consequently, an alternative catalytic triad was proposed comprising Thr12, Tyr25 and Glu294 (from the second subunit of the intimate dimer). Based on the available data, a twostep ping-pong mechanism has been finally put forward involving both Thr residues, each acting at a different stage of the catalytic reaction. In this case, L-ASNases appear to differ from other enzymes owing to the use of two catalytic triads instead of one [39].
Native and PEG L-ASPARAGINASE
L-asparaginase used for the therapy is available in three forms: two unmodified or native forms, purified from bacterial sources and one form modified from one of the native preparations. The native preparations are derived from E.coli and Erwiniacarotovora. The enzyme from both the sources has identical mechanism of action and toxicity but the pharmacokinetic properties differ. PEG-L-asparaginase is a chemically modified form of the enzyme in which L-asparaginase derived from E.coli is covalently conjugated to PEG. PEG-L-asparaginase is referred as pegasparaginase or pegasparagase, was developed in the 1970s and 1980s and was subjected to clinical trails in the 1980s which is approved by the food and drug administration for use as chemotherapy for the treatment of patients with ALL because of hypersensitivity to native form of E.coli L-asparaginase.
Native enzyme
L-asparaginase has been derived from different strains of E.coli [40]. The purified L-asparaginase from E.coli has a molecular weight of 133-141 kDa [41,42]. Asaparaginases consists of four subunits, each of molecular weight of around 22 kDa contains an active site[43].In Europe, two different preparations of E.coli L-aspraginase are available but with slightly different pharmacokinetic parameters [44]. L-asparaginase from almost all the organisms are known to contain around 10% glutiminase activity for which purification procedures are found to be difficult. Guinea pig serum is an exception but the amount of the drug obtained from this organism is not sufficient for clinical trials.
PEG L-Asparaginase
Native enzyme may cause hypersensitive reactions like allergic reactions due to high immunogenicity in 25% of the patients.Hence the modified one consists PEG attached to the therapeutic protein by covalent linkage to residues located at the surface of the native protein [16]. PEGylation of proteins usually results in masking of some surface sites, increasing the molecular size and enhancing steric hindrance. So, the native enzyme was chemically modified in order to reduce the immunogenicity not affecting the activity of the enzyme and increasing its half-life period in order to avoid frequent intra muscular injections [45, 46]. After so many efforts, finally in 1970s, PEGylation was found to be successful in preserving the antitumor activity reducing the immunogenicity. Abuchowski et al. [45] were first to successfully couple PEG to L-asparaginase which was tested for antitumor activity in the L5178Y tumor bearing BDF mouse model. Its biochemical properties differ from the native enzyme. The molecular weight is comparatively higher than native enzyme and its reactivity with specific antibodies is very low but increases when undergoes freeze-thawing cycles [44]. Clinical studies show that enzyme is having antitumor activity both in animal models [45, 47] and humans [47, 48]. The reduced Immunogenicity in vivo has been confirmed in highlysensitized children with multiple ALL relapses [45, 49]. Ettinger et al. [50] in 1994, reported absence of anaphylaxis, reduced incidences of hyperglycemia and pancreatitis duringthe treatment with polyethylene glycol-l-asparaginase. The comparison of commercially available native and modified enzymes are listed in (Table-1).
Table1: Comparison of native and modified L-Asparaginases.
| Characteristics | E. coli | Erwinia | |
| Native | PEG | Native | |
| Activity(IU/mg protein) 280- | 280 | 400 280 | 400 650 |
| Km(μM)- L-Asparaginase | 12 | 12 | 15 |
| Km(μM)- L-glutamine | 3,000 | 3,000 | 1,400 |
| Ratio maximal activity L-Gln/L-Asp | 0.03 | 0.03 | 0.10 |
| Molecular weight | 1,41,000 | 1,38,000 | |
| pH | 5.0 | 5.0 | 8.7 |
| Half-life (days) | 0.6 – 1.0 | 6.0 – 7.0 | 0.5 |
Different methods of chemical modifications were tried instead of having some disadvantages. Coupling with dextran was tried to improve thermal and proteolytic stability and to reduce immunogenicity but it was found to be less effective [51]. Coupling with poly-DL-alanyl peptides was tried to block the immunogenic epitoped but the clinical trials have not been yet performed [46]. Conjugation of L-asparaginase to human serum albumin was done in1989 for which the in vivo studies are still being done. Acylation was also tried which makes the enzyme hydrophobic after conjugation. Synthetic hexapeptide and polyclonal antisera from rabbits and mice was conjugated with the enzyme by site directed mutagenesis to eliminate immunodominant epitopes in Erwinia chrysanthemi. It leads to less immunogenicity without affecting its activity. This enzyme was expressed in E.coli and Erwinia carotovora which showed three-fold expression.
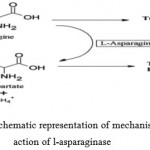 |
Figure 1: Schematic representation of mechanism of action of l-asparaginase.
|
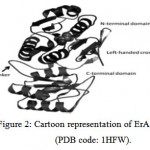 |
Figure 2: Cartoon representation of ErA monomer (PDB code: 1HFW). The large N-terminal domain and a smaller C-terminal domain connected by a linker of ~26 residues are labelled. The bound ligand (L-Glu) is shown in a space fill representation. |
Pharmacokinetics of the Drug
Pharmacokinetics of a drug depends upon route of administration and the type of preparation used. The route of administration may be either intramuscular or intravenous [52-54]. The half life of the enzyme from Erwinia and E.coli is similar, having 10h as mean half life. L-asparaginase after administration remains in the vascular space. It was found out that only small amount of L-asparaginase was detected in cerebrospinal fluid after intravenous administration of 5000 U/kg body weight. But the drug is immediately transferred to plasma when directly injected into the cerebrospinal fluid. The clinical pharmacology of the drug through intramuscular administration was studied and compared to intravenous route of administration. By injecting E.coli L-asparaginase into adults suffering from metastatic cancer. A lot of investments were made to find out the pharmacokinetic parameters. Chemically modified L-asparaginase is found to have longer half-life than native enzymes. PEGylation increases the half-life period which was investigated in number of patients having refractory malignancies, so, were heavily pretreated with the drug. Sometimes the half life decreases due to hypersensitivity to the drug found in some of the patients [55]. Many factors influence the antileukemic activity of asparaginases. Among these are:the biochemical factors of the rate of hydrolysis, and the km of the enzyme for asparaginase or glutiminase ; the pharmacological factor of serum clearance of the enzyme drug and the development of tumor cell resistance to asparaginase ;and the host immunological effects of anti-asparaginase antibody formation ,and augmented asparaginase “input” from the de novo biosynthesis of asparaginase .
Resistance to the Drug
The resistance to the drug is produced by depression of the aspargine synthetase gene in tumor cells. Aspargine synthetase is the enzyme responsible for the synthesis of l-aspargine in normal cells [56]. Pre-clinical and clinical tests conducted between L-asparaginase and cytosine arabinoside resulted in decrese in activity of asparagines synthetase and increased methylation of cytosine residues in the gene encoding this enzyme [57-59]. The asparagine synthetase activity increases in murine lymphoma cells and in patients suffering from ALL clinically resistant to the drug. The activity of asparagines synthetase increases with the increase in level of mRNA. The main reason for resistance is the accelerated clearance of L-asparaginase following induction of specific antibodies. The drugs efficacy remained unpaired even after immunization of patients. The development of antibodies influences the drugs action. There was another mechanism [60] described according to which the pool of L-asparginase produces cytokines that control the expansion of resistant cells. Resistant cells are escaped from regulatory control when sensitive cells are killed by L-asparaginase.
The major limitation of L-Asparaginase treatment in acute leukemia is the rapid development of clinical resistance. Accelerated clearance of L-Asparaginase by immunological mechanism has been reported in patients developing resistance. Another possible mechanism might involve the development of more efficient ways of utilizing L-asparagine, either from the plasma or from cells in which L-asparagine was not totally depleted by the L-Asparaginase treatment or in which L-asparagine was actively synthesized by asparagine synthetase. In human leukemic cells, the resistance is at least in part related to the asparagines synthetase activity. Before L-Asparaginase therapy, asparagine synthetase is undetectable in human leukemic cells. After therapy the cells from L-Asparaginase resistant patients showed a seven fold increase in asparagine synthetase [61].
Efficacy of the Drug
The antitumor specificity was investigated in animals and in cancer patients. Preclinical studies were found to be effective against more than 50 murine tumors which include rat and canine lymphosarcoma, rat fibrosarcoma, walker carcinoma and Jensen’s sarcoma [62]. Tumor of childhood and adult ALL is found to respond effectively and consistently during the clinical trials in cancer patients. But others were found to be inconsistent which were of lymphoid origin. The solid tumors were found to be resistant to the drug. L-asparaginase is found to improve event free survival when used during intensification phases of ALL treatment procedure, especially for the patients with high risk features at diagnosis including T-cell phenotype. L-asparaginase is found to be effective against meningeal leukemia also [63, 64]. Pegasparagase is used in reinduction and maintenance therapy. It is found to be more effective as it has more half life and can be taken once every two weeks. It is well tolerative against any hypersensitive reactions and shocks.
Toxicity of the Drug
The drug has a distinct toxicity profile, which varies from hypersensitivity to hepatocellular dysfunction [65]. Toxicity is cause due to either immunological reactions to a foreign protein or due to events taking place in the body due to inhibition of protein synthesis. The toxicity levels are almost similar for the enzyme from different preparations. L-asparaginase causes little bone marrow depression but does not affect the gastrointestinal tract or oral mucosa or hair follicles. It affects the liver and causes hypoalbumenia, elevations in transaminase, bilrubin and alkaline phosphatase, lipoprotein abnormalities and decreases the serum cholesterol. The enzyme causes acute hemorrhagic pancreatitis and decrease serum insulin in pancreas. It also affects central nervous system by creating mild depression and personality changes. It also leads to confusion and hallucinations. The hypersensitive reactions include localized erythema, indurations, edema, swelling, chill, fever, tenderness and skin rashes. These side- effects are increased due to number of factors like route of administration, amount of drug taken, etc. The hepatic functions returns to normal when intake of drug is stopped. There were some lethal thrombotic complications observed in the patients receiving multi-agent chemotherapy. These complications were less with PEG-L- asparaginase Formulation for an effective treatment. coupling of the enzyme to [poly (ethylene glycol)] or PEG was recognized as a process by which the immunogenic reactions of the drug were abolished without altering its antineoplastic activity. This modified version of the drug in animal models was observed to reduce the antibody formulation compared to its native form, and markedly prolonged the duration of its action. Many more chemical modifications, through certain advantages are tried like coupling with dextran, human serum albumin. Other possible combinations like with alkaloids, duanomycin destroy more leukemic cells than with either drug alone [66].
Clinical Trials with L-Asparaginase
The clinical trials of native L-asparaginases of Erwinia and E.coli were being discussed for past 30 years. The chemically modified enzyme has gained more importance in clinical applications for the treatment of leukemia due to the hypersensitive reactions caused with native preparation of the enzyme. PEGylated L-asparaginase is more safe than the native enzyme and has delayed plasma clearance activity that avoids the frequent intake of the drug [67,68]. During phase-I clinical trials, only three patients out of 31 adult patients showed toxic reactions like anaphylactic reactions, hyperglycemia and hepatic dysfunction when pegasparagadse was administered intravenously. Phase-I clinical tests and pharmacodynamic evaluations were done in the patients having solid tumors at advanced stages by administering pegasparagase. The amount of the drug given was varied biweekly which became successful in maintaining low levels of L-asparaginase for 14 days and some of the toxic reactions were observed after giving high amount of the drug. Phase-II study of pegasparagase in seven patients with refractory acute leukemias showed complete response in five of them and partial response in one of them. Randomized trial were designed to compare the safety, efficacy and feasibility of pegasparagase against native enzyme from E.coli.
The patients were subjected to pegaspargase doses of 250, 500, 1000, 1500 and 2000 U/m2 biweekly, which in majority of cases had been successful in maintaining low level of lasparagine for 14 days. Grade 1–2 hypersensitivity reactions were most frequently reported immunogenic toxicities at the 2000 U/m2 dose [69]. In a multicentre phase-II open label clinical trial (ASP- 201A), 21 patients with recurrent ALL were treated with single dose of pegaspargase (2000 IU/m2 every weeks) during an initial 14-day investigational window. Thereafter a standard multiagent chemotherapy consisted of vincristine and prednisone along with pegaspargase. Patients also underwent treatment with doxorubicine (40 mg/m2) and received intrathecal chemotherapy beginning on day 14. All had previously received l-asparaginase. During the 14-day investigational window with pegaspargasemonotherapy, 22% of the patients achieved complete or partial remission. The Pediatric Oncology Group no.8866 in comparative induction therapy of relapsed ALL treated patients with vincristine, prednisone (60 mg/m2 daily×28 doses), and used either native E. coli preparation (10,000 U/m2 per dose thrice aweek×12 doses) or pegaspargase (2500 U/m2 per dose fortnightly×two doses starting from day 1). The study gave almost identical results for combined complete response and partial response, which was 63% for pegaspargase and 65%in case of native preparation .In another randomized comparison between PEG and native-l-asparaginase of the Children’s Cancer Group (CCGstudy 1967), pegaspargase (2500 U/m2 ×single dose on day1) produced faster rate of remission than the native form (6000 U/m2 weekly×three doses). The overall complete response rates after 4 weeks were comparable, i.e. 98% for pegaspargase vis-a-vis 100% for the native drug [70]. An event free survival (EFS) was 80% for randomized patients during intensification multi-agent therapy at 5 years, 84±4%for native preparation (25,000 U/m2 weekly×30 doses) and78±4% for pegaspargase (2500 U/m2 biweekly×15 doses) treated patients [71].
Conclusion
It is concluded that L-asparaginase is an important therapeutic agent in the treatment of acute childhood lymphoblastic leukaemia (ALL) and lymphomas. Possibly the greatest importance of Asparaginase lies in the fact that it demonstrates the feasibility of attacking neoplastic cells on the basis of a specific nutritional requirement caused by the lack of a particular enzyme. The native L-Asparaginase was associated with immunogenic complications like mild allergic reactions to anaphylactic shocks and very short half-life value restrict subsequent therapy. Where as PEGylated L-asparaginase has also solved many complications found with native enzyme. It is very effective and safe and can be administered biweekly due to more half-life. It has undergone number of tests and found to maintain its activity even after less administration of the drug compared to the native enzyme. Recent advancements in technology have enabled detailed study of pharmacokinetic and pharmacodynamic of asparaginases from different sources which leads to more efficiency and designing of optimum dosing schedule for clinical applications. Future studies should examine additional aspects that would promote effective ASNase therapy, like the role of asparagine synthetase, gene expression profiles, and micro-array technology in order to categorize leukemia patients and search for inherent resistance or refractoriness to ASNase.
References
- Ortega JA, Nesbit Jr ME, Donaldson MH, et al. L-Asparaginase vincristine and prednisone for induction of first remission in acute lymphoblastic leukemia. Cancer Res 1977; 37: 535–40.
- Jones B, Holland JF, Glidewell O, et al. Optimal use of l-asparaginase (NSC-109229) in acute lymphoblastic leukemia. Med Pediatr Oncol 1977; 3:387–400.
- Clavell LA, Gelber RD, Cohen HJ, et al. Four-agent induction and intensive l-asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. New Engl J Med 1980; 315:657–63.
- Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose lasparaginase improves survival for pediatric patients with T-cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia 1999; 13:335–42.
- Schrappe M, Reiter A, Ludwig W, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines cranial radiotherapy: results of trial ALL-BFM 90. Blood 2000;95:3310–22.
- Hann I, Vora A, Richards S, et al. Benefit of intensified treatment for all children with acute lymphoblastic leukemia: results from MRC UKALL XI and MRC ALL97 randomized trials. Leukemia 2000; 14:356–63.
- Gokbuget N, Hoelzer D. Recent approaches in acute lymphoblastic leukemia in adults. Rev Clin Exp Hematol 2002;6(2):114–41 [discussion 200–202].
- Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood 1995;85(8):2025–37.
- Ertel I, Nesbit M, Hammond D, Weiner J, Sather H. Effective dose of l-asparaginase for induction of remission in previously treated children with acute lymphoblastic leukemia: a report from Children’s Cancer Study Group. Cancer Res 1979; 39:3893–6.
- Rizzari C, Citterio M, Zucchetti M, et al. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica 2006; 91:24–31.
- Nesbit M, Chard R, Evans A, Karon M, Hammond G. Evaluation of intramuscular verses intravenous administration of l-asparaginase in childhood leukemia. Am J Pediatr Hematol Oncol 1979;1:9–13
- Ashihara Y, Kono T, Yamazaki S, Inada Y. Modification of colil-asparaginase with polyethylene glycol: disappearance of bindingability to anti-l-asparaginase serum. Biochem Biophys Res Commun1978; 83:385–91.
- Park YK, Abuchowski A, Davis S, Davis F. Pharmacology of coli l-asparaginase polyethylene glycol adduct. Anticancer Res1981; 1:373–6.
- Clementi A. La desemidation enzymatique de l-asparagine chez les differentes especes animales et la signification physiologique de sa presence dans l organisma. Arch Int Physiol 1922; 19:369–76.
- Kidd J. Regression of transplanted lymphoma induced in vivo by means of normal guinea pig serum I. J Exp Med 1953; 98:565–82.
- Kidd JG. Regression of transplanted lymphomas induced in vivo by means of normal Guinea pig serum II. J Exp Med 1953; 98:583–606.
- Neuman RE, McCoy TA. Dual requirement of Walker carcinosarcoma 256 in vitro for l-asparagine and glutamine. Science 1956; 124:124–31.
- Haley EE, Fischer GA, Welch AC. The requirement for L-asparagine of mouse leukemia cells L5178Y in culture. Cancer Res 1961; 21:532–41.
- Broome JD. Evidence that the l-asparaginase activity in guinea pig serum is responsible for its antilymphoma effects. Nature 1961; 191:1114–5.
- Broome JD. Evidence that the l-asparaginase of guinea pig serum is responsible for its antilymphoma effects I. J Exp Med 1963; 118:99–120.
- Yellin TO, Wriston JC. Purification and properties of guinea pig serum l-asparaginase. Biochemistry 1966; 5:1605–12.
- Yellin TY, Wriston JC. Antagonism of purified asparaginase from guinea pig serum towards lymphoma. Science 1966; 151:998–1004.
- Mashburn L, Wriston JC. Tumor inhibitory effects of l-asparaginase from Escherchia coli. Arch Biochem Biophys 1964; 105:450–2.
- Campbell H, Mashburn L. l-Asparaginase EC-2 from Escherchia coli some substrate specificity characterstics. Biochemistry1969; 9:3768–75.
- Oettgen RF, Old JL, Boyse EA, et al. Inhibition of leukemias in man by l-asparaginase. Cancer Res 1967; 27:2619–31.
- Duval M, Suciu S, Ferster A, et al. (2002) Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer, Children’s Leukaemia Group phase 3 trial., , 99, 2734–2739.
- Yurel, E.; Peru, D.; Wriston, J.J. On the distribution of plasma L-asparaginase. Experientia, 1983, 39, 383-385.
- Michalska, K.; Jaskolski, M. Structural aspects of L-asparaginases, their friends and relations. Acta Biochim. Pol., 2006, 53, 627-640
- Lubkowski, J.; Wlodawer, A.; Housset, D.; Weber, I.T.; Ammon, H.L.; Murphy, K.C.; Swain, A.L. Refined crystal structure of Acinetobacter glutaminasificans glutaminase-asparaginase. Acta Crystallogr. D, 1994, 50, 826-832.
- Mishalska, M.; Bujacz, G.; Jaskolski, M. Crystal structure of plant asparaginase. Mol. Biol., 2006, 360, 105-16.
- Tarentino, A.L.; Maley, F. The purification and properties of a beta-aspartyl N-acetylglucosylamine amidohydrolase from hen oviduct. Biochem. Biophys., 1969, 130, 295-303
- Ortuno-Olea, L.; Duran-Vargas, S. The L-asparagine operon of Rhizobium etli contains a gene encoding an atypical asparaginase. FEMS Microbiol. Lett., 2000, 189, 177-182.
- Shimizu T, Kubota M, Adachi S. (1992) Pretreatment of a human T lymphoblastoid cell line with L-Asparaginase reduces etopsideinduced DNA strand breakage and cytotoxicity., Int J Cancer., 50, 644–648
- Swain, A.L.; Jaskólski, M.; Housset, D.; Rao, J.K.; Wlodawer, A., Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Natl. Acad. Sci. USA, 1993, 90, 1474- 1478.
- Yun, M-K.; Nourse, A.,; White, S.W.; Rock, C; Richard J. Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli L-asparaginase I. Mol. Biol., 2007, 369, 794-811.
- Miller, M.; Rao, J.; Wlodawer, A.; Gribskov, M., A left-handed crossover involved in amido hydrolase catalysis. Crystal structure of Erwinia chrysanthemi L-asparaginase with bound L-aspartate. FEBS Lett., 1993, 328, 275-279.
- Aghaiypour, K., Wlodawer, A., Lubkowski, J., (2001). Structural basis for the activity and substrate specificity of Erwinia chrysanthemi L-Asparaginase., Biochemistry., 40, 5655–5664.
- Ortlund, E.; Lacount, M.W.; Lewinski, K.; Lebioda, L. Reactions of Pseudomonas 7A glutaminase-asparaginase with diazo analogues of glutamine and asparagine result in unexpected covalent inhibitions and suggests an unusual catalytic triad Thr-Tyr-Glu. Biochemistry, 2000, 39, 1199-1204.
- Dodson, G.; Wlodawer, A. Catalytic triads and their relatives. Trends Biochem. Sci., 1998, 23, 347-352.
- Irion E, Arens A. Biochemical characterization of L-asparaginase from coli. In: Grundmann E, Oettgen HF, editors. Experimental and Clinical effects of l-asparaginases. RRCR, Heidelberg: Springer; 1979. p. 39–57.
- Jackon RD, Handschumacher RE. Escherchia coli L-asparaginase: catalytic activity and subunit nature. Biochemistry 1970; 9:3585–90.
- Maita T, Matsuda G. The primary structure of l-asparaginase from Escherchia coli. Hoppe seyler’s Z Physiol Chem 1980; 361:105–17.
- Whelan H, Wriston H. Purification and properties of asparaginase from Escherchia coli. Biochemistry 1969; 8:2386–93.
- Korholz D, Bruck M, Nurnberger W, et al. Chemical and immunologicalcharacterisitics of four different l-asparaginase preparations.Eur J Hematol 1989;42:417–24.
- Abuchowski A, Van Es T, Palczuk NC. Treatment of L5178Y tumor bearing BDf mice with a non-immunogenic L-Glutaminase asparaginase. Cancer Treat Rep 1979; 63:1127–9.
- Uren JR, Ragin RC. Improvement of therapeutic immunologicaland clearance properties of coli and Erwinia caratovora L-asparaginasesby attachment of poly-dl-alanyl peptides. Cancer Res1979; 39:1927–33.
- Yashimoto T, Nishimura H, Saito Y. Characterization of PEG modified l-asparaginase from coli and its application to the therapy of leukemia. Jpn J Cancer Res 1986; 77:1264–71.
- J¨urgens H, Schwamborn D, Korholz D, et al. Klinische Erfanrungen mit polyathylengekoppelter E. coli Asparaginase bei patienten mit ALL. Mehrfachrezidiv Klin Padiatr 1988; 200:184–91.
- McEwen EG, Rosenthal R, Matus R, et al. A preliminary study on the evaluation of asparaginase. Cancer 1987; 59:2011–20.
- Ettinger LJ, Kurtzberg J, Voute PA, Jurgen H, Halpern HL. An open label, multicentre study of polyethylene glycol l-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer 1995; 75:1176–81.
- Davis FF, Kazo GM, Nucci ML, Abuchowski A. Reduction of immunogenicity and extension of circulating half-life of peptides and proteins. In: Lee VHL, editor. Peptide and protein drug delivery. New York: Marcel Dekker; 1991. p. 831–51.
- Asselin B, Whitin J, Coppola D, Rupp I, et al. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol 1993; 11:1780–6.
- Ho DH, Brown NS, Yen A, et al. Clinical pharmacology of polyethylene glycol-l-asparaginase. Drug Metab Dispos 1986; 14:349–52.
- Albertsen BK, Jakobsen P, Schroder H, Schmiegelow K, Carlsen NT. Pharmacokinetics of Erwinia l-asparaginase after intravenous and intramuscular administration. Cancer Chemother Pharmacol 2001; 48:77–82.
- Keating MJ, Holmes R, Lerner S, Ho DH. L-Asparaginase and PEG-asparaginase-past, present, and future. Leuk Lymphoma 1993; 10(Suppl.):153–7.
- Andrulis IL, Barrett MT. DNA methylation patterns associated with asparagine synthetase expression in asparagine overproducing and auxotrophic cells. Mol Cell Biol 1989; 9:2922–30.
- Nyce J. Drug induced DNA hypermethylation and drug resistance in human tumors. Cancer Res 1989; 49:5829–36.
- Schwartz S, Morgenstern B, Capizzi R. Schedule dependent synergy and antagonism between high-dose 1-_-d-arabinofuranosylcytosine and asparaginase in the L 5178Y murine leukemia. Cancer Res 1982;42:2191–7
- Yap B, McCredie K, Benjamin R, Bodey G, Freireich E. Refractory acute leukemia in adults treated with sequential collaspase and high dose methotrexate. BMJ 1978;2:791–3
- Gallagher MP, Marshall RD, Wilson R. l-Asparaginase a drug for treatment of acute lymphoblastic leukemia. Essays Biochem 1989;24:1–40.
- Jaffe N, Traggis D, Das L, et al. (1971) Favourable remission induction rate with twice week doses of L-Asparaginase., Cancer Res., 31, 1–4.
- Winston JJ, Yellin T. l-Asparaginase: a review. Adv Enzymol 1973;39:185–248
- Bushara KO, Rust RS. Reversible MRI lesions due to pegaspargase treatment of non-Hodglein’s lymphoma. Pediatr Neurol 1997; 17:185–7.
- Hill J, Loeb E, McLellan A, et al. Responses to highly purified l-asparaginase during therapy of acute leukemia. Cancer Res 1969; 29:1574–80.
- Oettgen HF, Stephenson PA, Schwartz MR, et al. Toxicity of coli l-asparaginase in man. Cancer 1970;25:253–78
- Boos J,Werber G, Ahlke E, et al. (1996) Monitoring of L-Asparaginase activity and asparaginase levels in children on different L-Asparaginase preparations., 32A, 1544–1550
- Kurtzberg J, Friedman H, Asselin B, et al. The use of polyethylene glycol-conjugated l-asparaginase (PEG-Asp) in pediatric patients with prior hypersensitivity to native l-asparaginase. Proc Am Soc Clin Oncol 1990; 9:219.
- Ettinger L, Asselin B, Poplack D, Kurtzberg J. Toxicity profile of PEG-l-asparaginase in native l-asparaginase-hypersensitive and monhypersensitive patients with acute lymphoblastic leukemia (ALL). In: Presented at international Society of Pediatric Oncology (SIOP) 25th meeting. 1993.
- Taylor CW, Dorr RT, Fanta P, Hersh EM, Salmon SE. A phase I and pharmacodynamic evaluation of polyethylene glycol-conjugated l-asparaginase in patients with advanced solid tumors. Cancer Chemother Pharmacol 2001; 47:83–8.
- Holcenberg J, Sencer S, Cohen LJ, et al. Randomised trial of PEG vs. native l-asparaginase in children with newly diagnosed acute lymphoblastic leukemia (ALL): CCG study 1962. Blood 1999; 94:628a.
- Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol. 91–01. Blood 2001; 97:1211–8.

This work is licensed under a Creative Commons Attribution 4.0 International License.





