Manuscript accepted on : 18 November 2011
Published online on: --
Endocrine - Disrupting Carcinogenic Plastic Contamination in the Food Chain : A Review
Tarun Batra
Voice for Earth International 225, Cedar Hill Rd, Suite 200, Marlborough, MA 01752 USA.
ABSTRACT: The breakdown products from unrecycled and improperly disposed plastics in land and at sea affect the aquatic organisms in a functional and developmental level. The control of liquid leachates and gaseous emissions has become necessary as new classes of pollutants from plastics are discovered. While these pollutants may be quickly metabolized by most biota, the continuous release of low levels of pollutants coupled with bio- magnification in higher tropic levels bears further study on the potential effects of these breakdown products and their dispersal within the food chain. There are documented effects of these leachates as endocrine disruptors or as carcinogens. There are studies on evidence of concentrations of BPA, DEHP or other plasticizers in fish tissues as well as in freshwaters and other marine environment, mostly in surface waters and sediments. As the use of plastics continues and expands with an increasing consumption of plastics in developed and developing countries, it is vital to find novel ways to remove plastics from landfills or marine environment, failing which varied endocrine disruptors and carcinogens will have undesirable effects on ecosystems, native aquatic organisms and the human populations who consume them.
KEYWORDS: Plastic Leachates; BPA; Human Food chain Contamination; Endocrine Disruptors
Download this article as:| Copy the following to cite this article: Batra T. Endocrine - Disrupting Carcinogenic Plastic Contamination in the Food Chain : A Review. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Batra T. Endocrine - Disrupting Carcinogenic Plastic Contamination in the Food Chain : A Review. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9448/ |
Introduction
The disposal of solid waste on both land and at sea results in the creation of breakdown products from plastics that affect the organisms in a functional and developmental level. While the breakdown products from plastics have been less studied in comparison to previous research on pesticides and heavy metals, these contaminants present a modern hazard to ecosystems. As communities and regions become more prosperous in the developing world, more plastics are used as components of cosmetics, clothing, and containers for food. The normal life cycle of these consumer products includes an endpoint of disposal or recycling. In the case of plastic disposal in a landfill, mechanical and chemical breakdown will occur resulting in release of constituents to the environment (Teuten et al, 2009). Ultimately more research is needed to determine the scale of effect these breakdown products have in the natural environment.
The main classes of plastics that generate breakdown products of concern are polyvinyl chloride (PVC) and polycarbonates. Vinyl chloride, the chemical used to make PVC is known to be a carcinogen, and the plasticizers (phthalates) used to make PVC flexible are believed to be endocrine disruptors or have estrogenic activity. The primary building block of polycarbonates, Bisphenol-A (BPA) [ Figure I ] is an estrogen-like endocrine disruptor (Harris et al). Unreacted BPA monomer releases from polycarbonates containers quite easily under wet conditions, thus, leachates from plastics disposed of in solid waste landfills include phthalates Bis (2-ethylhexyl) phthalate (DEHP) [ Figure II ] , diethyl phthalate (DEP) [ Figure III ] , and BPA.
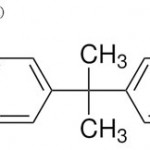 |
Figure 1: Bisphenol-A (BPA).
|
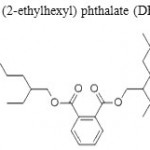 |
Figure 2: Bis (2-ethylhexyl) phthalate (DHEP.
|
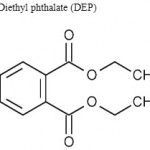 |
Figure 3: Diethyl phthalate (DEP).
|
Land based disposal of solid waste in the United States (US) alone accounts for approximately 253 million tons of solid waste per year (Rhind, 2009). Modern solid waste landfills seek to contain these solid and liquid breakdown products within the landfills with a combination of landfill linings, leachate treatment systems, and ventilation systems. The control of liquid leachates and gaseous emissions has become necessary as new classes of pollutants from plastics are discovered. While these pollutants may be quickly metabolized by most biota, the continuous release of low levels of pollutants coupled with bio- magnification in higher trophic levels bears further study. The US Environmental Protection Agency (EPA) and other agencies have collected data over the past twenty years concerning the effects of these breakdown products and their dispersal within the food chain. The effects of the leachates may be two-fold; they may have an effect as an endocrine disruptor, or an effect as a carcinogen. Endocrine disruptors are chemicals known to have an interfering effect on the hormone system in animals (Harris, et al, 2010; Aluru, et al., 2010). The primary disruptions within the hormone systems are most apparent in animals with a lower mass and also in younger animals during crucial periods of rapid growth and developmental changes. Secondarily, the disruption of hormone systems can interfere with learning, sexual development, and accelerate tumor growth. Carcinogens by contrast directly affect tissues and disrupt the genetic material of cells or their metabolic processes that leads to the development of cancer. Cells that do not die from the cell damage caused by carcinogens may divide in an aggressive or uncontrolled manner to form tumors.
The phthalate DEHP is used effectively as a plasticizer in PVC compounds, and as a chemical is very soluble in oil (polar solvents) but only poorly so in water. DEHP releases from PVC when it is heated and unreacted molecules of DEHP either outgas or are released into solution. Thus, the Food and Drug Administration (FDA) does not allow DEHP-containing packaging to be used for food other than those packaged in water. DEHP metabolizes to mono-ethylhexyl phthalate (MEHP) and then to phthalate salts. Research on DEHP indicates a correlation with insulin resistance (a diabetes precursor), changes in male genital formation (prenatal) and during development (antenatal), and interference with cardio cell functioning (in cultured cells). Due to these issues, many countries have banned use of DEHP in the manufacture of products that come in contact with human skin, toys, and food containers.
The compound BPA is used commonly in the manufacture of polycarbonates and epoxy resins. BPA has been known to have estrogenic effects since the 1930’s, and growing concerns since 2008, have resulted in a ban from its use in baby bottles in European Union and Canada since September 2010. BPA acts as an estrogenic endocrine disruptor, with potential prenatal exposure leading to potential neurological difficulty. While BPA is believed to be rapidly metabolized and eliminated, the continuous low dosage of this chemical leads to concerns on human health effects.
A recent study of BPA concentrations in leachates from municipal waste landfills in tropical Asia sought to identify the relationship between economic development and BPA in leachates. The results ranged from sub microgram per liter (µg/l) to milligrams per liter (mg/l), and correlated well with the regional level of economic development. Leachate concentrations from US, German, and Japanese landfills are identified as 10-10000 µg/l (Teuten et al, 2009). This is apparently up to seven orders higher than the no effect concentration (NOEC) of BPA for endocrine disruptors in freshwater organisms (Schulte-Oehlmann et al, 2001), and one to five times higher than the levels of BPA regularly found in wastewater treatment effluent. This study identified the importance of recognizing the contributions of leachates as a source of BPA and other breakdown products in aquatic environments. Additionally, rising BPA concentrations were identified as more problematic for areas/regions, where there are no, or poorly functioning leachate treatment containment or treatment. Acidogenic leachates release occurs during the early landfill phase when additive or unreacted BPA will release under acidified conditions, as does DMP phthalate (or other more hydrophilic leachates). During organic breakdown in later landfill phase, methanogenic leachates form and cause a more prevalent release of hydrophobic leachates such as DEHP.
While sediment concentrations of phthalate pollutants generally are consistent with urban sources, studies of fish tissue from those same sites do not always correlate. A study in the 1990’s found high levels of organic sediment contaminants in the Tualatin Watershed in Oregon, where the Beaverton Creek site had higher levels of contaminants all other sites sampled in the study (Bonn, 1999). This site was one of two that had an exceedence of a USEPA Tier 1 screening value for DEHP (screening value of 2.65 µg/g). By contrast, phthalates were not detected in sediments at background sites (Gales Creek and Tualatin River above Dairy Creek, Oregon). Despite the high concentrations of phthalates in sediments, no correlation of phthalate concentrations in fish tissue was found. This may have been due to the relatively low numbers of fish samples taken, rapid metabolism of DEHP, or DEHP concentrations being lower than able to be analyzed with the tests used.
Historically, few studies of concentrations of BPA, DEHP or other plasticizers in fish tissue were conducted in the 1980s or 1990s. Contaminants of concern primarily focused on organo-chlorine pesticides and heavy metals. However, one study of fish collected from Great Lakes tributaries in Wisconsin and Ohio, during 1981 identified DEP in composite whole-body tissue samples at concentrations ranging from <0.02 milligrams per kilogram (mg/kg) to <0.30 mg/kg (DeVault, 1985). Lake trout (Salvelinus namaycush) and whitefish (Coregonus clupeaformis) taken from Lake Superior near Isle Royale, Michigan, USA, had elevated levels of DEP (0.5 and 2.2 µg/g, respectively) compared with lake trout and whitefish taken from other parts of Lake Superior (both values below the level of quantification of 0.001 µg/g wet weight). Fish taken from Siskiwit Lake on Isle Royale, Michigan, an area supposedly unaffected by human activity, also had relatively high concentrations of DEP in their tissue, 0.4 mg/kg for lake trout and 1.7 mg/kg for whitefish (Sekizawa).
More recent studies in Sweden and elsewhere have demonstrated the presence of BPA in freshwaters as well as in the marine environment, mostly in surface waters and sediments. A Swedish screening study of BPA performed in 2003-2004 showed that BPA was detectable in a number of matrices. For instance, BPA was ubiquitously occurring in sludge from municipal sewage treatment plants, with levels generally less than 1 mg/kg dry weight. It was occasionally found in surface sediments. Perch from 49 sites were analyzed but only four samples contained detectable levels (i.e. > 20 nanograms per gram [ng/g] wet weight). Particulate BPA was also detected in air at the ng/cubic meter level at several urban sites. Very few data exists on the occurrence of BPA in biota, but levels around 1 ng/g wet weight in fish have been documented from Norway and the Netherlands. In order to strengthen the knowledge on the environmental occurrence of BPA in Sweden, further study was undertaken in a study of BPA analyzed in fish from background lakes, urban lakes, coastal sites and the marine environment (Sternbeck, 2007). In total 23 samples were analyzed. Bisphenol-A was detected in most samples in the range of <0.24 to 4.7 (ng/g) wet weight in muscle. Liver from cod caught outside Gotland displayed concentrations in the range <0.24-1.77 ng/g wet weight. There were no pronounced differences in concentrations between different species or when urban sites were compared to background sites. With respect to possible risks for toxicological effects on fish or their consumers (e.g. humans), the concentrations found in in the study were regarded as low.
Persson et al. (1978) collected aquatic organisms from the vicinity of a DEHP factory in Finland. Invertebrates contained up to 0.1 mg DEHP/kg, and levels of 1.1 mg/kg in roach muscle and 2.3 mg/kg in pike liver were measured. Thuren (1986) analyzed biota near an industrial discharge point and reported levels of up to 5.3 mg/kg (fresh weight) in insects including Odonata species and up to 14.4 mg/kg in Asellus aquaticus. Studies have indicated bottom feeding catfish DEHP concentrations of 400-3200 ug/kg (Mayer eta al 1972). Musial & Uthe (1980) collected fish of various species from the Gulf of St Lawrence, Canada, and analyzed them for DEHP in lipid extracts. They reported levels of up to 6.5 mg/kg on a wet weight basis (51.3 mg/kg on fat weight basis) in mackerel muscle and up to 7.2 mg/kg (47.1 mg/kg fat weight) in herring muscle. Lower levels of 0.37 mg/kg (wet weight) were found in eels, and in both plaice and redfish concentrations were less than 0.001 mg/kg. In Japan, DEHP levels in various species of fish in rivers and seas ranged from 0.01 to 19 mg/kg wet weight in 1974 (Environmental Agency of Japan, 1989).
Despite low concentrations of pollutants in sediment or water, bioaccumulation due to the ingestion of planktivorous fish by predators (commercial/game fish) may occur in natural populations. The resulting retention within tissue may result in bio-magnification. Studies of bioaccumulation of BPA and phthalates are few and vary widely in result, indicating the possibility of a wider range of expected bioaccumulation in different species. Since dibutyl phthalate (DBP) is readily metabolized in fish, bioaccumulation should be limited in fish species. The limited data that are available however, fail to confirm this however, as the reported bio-concentration factors for DBP for various aquatic organisms ranges from 2.9 for the brown shrimp, Penaeus aztecus, to 2125 for the fathead minnow, Pimephales promelas (Heise and Litz, 2004). This variance would indicate that further research concerning bioaccumulation of phthalates and BPA in the wild aquatic ecosystem is necessary to evaluate the scale of potential contamination of the human food chain through ingested organisms.
As the use of plastics continues and expands with the world’s expanding population and the growth of a wealthier consumer class in developing countries, novel ways to recycle or remove plastics from landfills should be found. DEHP, BPA, DMP, and DEP are but a few of the endocrine disruptors that may have undesirable effects on ecosystems. Ultimately in areas with poor environmental controls, these plastic pollutants will probably be found to have a greater effect on native aquatic organisms and the human populations who consume them.
References
- Bonn, Bernadine A., Selected Elements and Organic Chemicals in Bed Sediment and Fish Tissue of the Tualatin River Basin, Oregon, 1992-96. Water-Resources Investigations Report 99-4107. Portland, Oregon, 1999.
- Catherine A. Harris, Patrick B. Hamilton, Tamsin J. Runnalls, Veronica Vinciotti, Alan Henshaw, Dave Hodgson, Tobias S. Coe, Susan Jobling, Charles R. Tyler, John P. Sumpter. The Consequences of Feminisation in Breeding Groups of Wild Fish. Environmental Health Perspectives, 2010; DOI:
- Heise, Stephan, and Norbert Litz. Phthalates Deskstudy. German Federal Environmental Agency, 2004
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, O.; Wollenberger, L.; Santos, M. et al. (Jul 2009). A critical analysis of the biological impacts of plasticizers on wildlife. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 364 (1526): 2047–2062.
- Neelakanteswar Aluru, John F. Leatherland, John, and Vijayan, Mathilakath. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. Plosone. 2010, May 20.
- S.M. Rhind, Anthropogenic pollutants: A threat to ecosystem sustainability. Philosophical Transactions of the Royal Society of Biological Science. November 2009 Volume 364, pp. 3391-3401.
- Sternbeck, John, Screening of bisphenol-A in fish from Swedish waters. Naturvardsverket. October 2007.
- Teuten, E, J.M. Saquing, D.R.U. Knappe, M.A. Barlaz, S. Jonsson, Annika Bjorn, Steven J. Rowland, Richard C. Thompson, Tamara S. Galloway, Rei Yamashita, Daisuke Ochi, Yutaka Watanuki, Charles Moore, Pham Hung Viet, Touch Seang Tana, Maricar Prudente, Ruchaya Boonyatumanond, Mohamed P. Zakaria, Kongsap Akkhavong, Yuko Ogata, Hisashi Hirai, Satoru Iwasa, Kaoruko Mizukawa, Yuki Hagino, Ayako Imamura, Mahua Saha, and Hideshige Takada. Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society of Biological Science. July 2009, volume 364, pp. 2027-2045.

This work is licensed under a Creative Commons Attribution 4.0 International License.





