Manuscript accepted on : January 08, 2011
Published online on: 28-06-2011
Adel A. Razek, Adel A. Shalaby, El Sadek A. Maaly and Yasser E. Elazhary
Department of Ophthalmology, Zagazig University, M.D. , Zagazig - 44519 Egypt.
ABSTRACT: Purpose To compare the outcomes of phaco-deep scelerectomy (P-DS) augmented with mitomycin C with phaco-trabeculectomy (P-T) principally regarding the effect on the intraocular pressure (IOP). Methods Fifty eyes of thirty-eight patients diagnosed with senile cataract and primary open angle glaucoma were divided into two equal groups. Group A underwent Phaco-deep sclerectomy (PDS) with mitomycin C (MMC) while group B underwent phaco-trabeculectomy (PT). Postoperative IOP was followed up for six months. Results By the end of the sixth month complete success (IOP < 21mmHg without anti-glaucoma therapy) was achieved in 80% in group A and 84% in group B. By the end of the sixth month qualified or partial success (IOP < 21mmHg with anti-glaucoma therapy) was achieved in 12% in both groups A and B. Conclusion P-DS with MMC does not exceed conventional P-T in what concerns IOP reduction. However the complication rate is lower in P-DS. Augmentation of PDS with MMC is safe with few serious complications related to its use and MMC augmentation appears to increase the probability of achieving lower target intraocular pressures after combined P-DS.
KEYWORDS: Combined surgery-Deep sclerectomy-Mitomycin C-Phacoemulsification- Trabeculectomy
Download this article as:| Copy the following to cite this article: Razek. A. A, Shalaby. A. A, Maaly. E. S. A, Elazhary. Y. E . Phaco-Deep Sclerectomy With Mitomycin C Versus Phaco-Trabeculectomy in Patients With Coexisting Cataract and Glaucoma. Biosci Biotechnol Res Asia 2011;8(1) |
| Copy the following to cite this URL: Razek. A. A, Shalaby. A. A, Maaly. E. S. A, Elazhary. Y. E . Phaco-Deep Sclerectomy With Mitomycin C Versus Phaco-Trabeculectomy in Patients With Coexisting Cataract and Glaucoma. Biosci Biotechnol Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9193 |
Introduction
The existence of cataract and glaucoma in the same eye requires special consideration when surgical intervention is needed. If filtering surgery is done first, subsequent cataract surgery may jeopardize an already functioning filtering bleb. If cataract surgery is done first, postoperative elevation of intraocular pressure (IOP) may occur and threaten further loss of visual field.1
Surgical management of a patient with coexisting cataract and glaucoma has changed as the use of phacoemulsification has increased. The advantages of small incision cataract surgery include decreased operating time, more rapid return to full physical activity, earlier stabilization of refraction, and less induced astigmatism. Reduced conjunctival dissection and manipulation minimizes inflammation and reduce subsequent bleb scarring. In addition, more virgin conjunctiva is preserved in the event that further glaucoma surgery is required. These advances have led to an increased enthusiasm for combining cataract and glaucoma surgery, and many surgeons have expanded their indications for combined surgery as opposed to a two-stage approach.2
Although trabeculectomy is currently regarded as the standard surgical procedure combined with cataract surgery3, it can cause undesirable postoperative complications including hyphema, excessive filtration leading to shallow or flat anterior chamber, choroidal detachment, hypotonymaculopathy, suprachoroidalhaemorrhage, bleb-related problems, and increased risk of endophthalmitis.4
Non-penetrating glaucoma surgery, including deep sclerectomy, is a viable alternative to trabeculectomy with advantages of decreased postoperative complications. This procedure can be combined with phacoemulsification in managing patients with coexisting cataract and glaucoma with very satisfactory results with regard to visual outcome and IOP control.5
Patients and Methods
50 eyes of 38 patients were included in this study. All patients were diagnosed as having significant senile cataract and POAG with intraocular pressure above 21 mmHg on maximally tolerated therapy (two or more eye pressure-lowering drugs). Patients with primary angle closure glaucoma, secondary angle closure glaucoma and secondary open angle glaucoma were excluded from this study.
The patients were divided into two groups: Group (A): 25 eyes underwent phacoemulsification with intraocular lens (IOL) implantation and deep sclerectomy with intraoperative mitomycin C augmentation (PDS). Group (B): 25 eyes underwent phacoemulsification with IOL implantation and trabeculectomy (PT). General anaesthesia was performed in most of the cases. Phacoemulsification was done first followed by the glaucoma operation in different two sites (two-site procedure). A 3 mm keratome was passed into the temporal clear. Viscoelastic material was then injected to fill the anterior chamber. Two paracenteses, approximately at right angle to the corneal incision, were performed using a 15-degree keratome. A continuous tear capsulorrhexis was then made, under viscoelastic agent, starting with a bent insulin syringe then completed with the capsulorrhexis forceps. Hydrodissection was then performed. Phacoemulsification of the nucleus was then started; the most commonly technique used was stop and chop. After phacoemulsification, I/A was done. Viscoelastic was then injected and foldable IOL was implanted. The eye was kept filled with viscoelastic and then glaucoma operation was started. A fornix-based 7mm conjunctival flap was performed. In cases of phaco-deep scelerectomy (P-DS), a superficial scleral flap—a third of the estimated total scleral thickness, measuring 5 x 5 mm—was delineated then dissected (fig.1) forward into the clear cornea for 1-1.5 mm. A sponge soaked with MMC concentration 0.4mg/ml was put under the scleral flap (fig.2) for 3 minutes. Irrigation of the scleral bed and sub conjunctival space was then done very thoroughly. At this stage, magnification was increased and maximum illumination was used to start dissection of the deep scleral flap with an assistant holding the scleral flap with fine-toothed forceps, to allow good exposure of the scleral bed for the deep scleral dissection. The deep scleral flap measuring 4×4 mm. leaving a margin of 0.5 mm on each side was dissected 90-95% of the thickness of the bed with a surgical 15 Bard Parker knife (fig.3). Anteriorly, the dissection was made to Schlemm’s canal which was unroofed (fig.4), more anteriorly the corneal stroma was excised to Descement’s membrane. Deep scleral flap was excised (fig.6) leaving behind the filtration membrane, termed trabeculo-Descement’s membrane (TDM) (fig.5) through which aqueous humor was seen percolating. If percolation was inadequate, further stripping was needed but perforation of the window was a potential risk. When this occurred, the procedure was completed as trabeculectomy with internal block dissection and peripheral iridectomy but these patients were excluded from the study, and replaced. The superficial scleral flap was loosely sutured with two 10/0 nylon sutures, the knots are buried. Bimanual I/A was then performed to remove the viscoelastic. The conjunctival ends were sutured with inverted 10/0 nylon sutures. One mattress transverse suture was taken suturing conjunctiva to the limbus. This was followed by subconjunctival injection of gentamycin and dexamethasone. In cases of PT, a 4-5 mm wide scleral half thickness incision was done 3 mm behind the anterior limbal vascular archades with a surgical 15 Bard Parker knife; the flap was dissected anteriorly, extending about 1 mm into clear cornea. Trabeculectomy was done using the 15-degree keratome. Vertical incisions on both sides of the trabeculum were done first (fig.7), then a horizontal incision was done using the 15-degree keratome. The trabecular block was then removed on vision using Vannas scissor (fig.8), while scleral flap was retracted. The iris root was then grasped with a Colibri forceps, pulled out of the wound, performing peripheral iridectomy by Vannas scissor (fig.9). Reposition of the trap door and 10/0 nylon sutures are taken. All patients received post-operative therapy in the form of systemic levofloxacin 500 mg once per day for five days. Topical treatment was used in the form of combined steroid-antibiotic eye drops five times per day and ointment once at night for a week. Cases were examined 24 hours postoperatively then after 2 days then weekly during the first post-operative month then monthly for six months. During each visit, the I.O.P was measured by applanation tonometry. The bleb was examined (height, extent, colour, vascularity). BCVA was measured. Anterior segment was examined for any abnormalities (Flare, cells, IOL position &capsular opacification …). Gonioscopy was done when needed to exclude obstruction of trabeculectomy site or iridectomy. Success was defined as: (a) Complete success: IOP less than 21mmHg without any anti-glaucoma medications. (b) Qualified success: IOP less than 21mmHg with one or two topical anti- glaucoma medications. (c) Failed cases: IOP more than 21mmHg with two topical anti- glaucoma medications and the need of another surgery.
Table 1: Comparison between the two groups concerning change in IOP using test.
| Group | Group A | Group B | t | P value |
| IOP | Mean ± SD | Mean ± SD | ||
| Pre op. | 30.68 ± 4.16 | 30.72 ± 3.92 | 0.035 | 0.97 |
| Post op. 1w | 9.90 ± 1.62 | 10.34 ± 1.50 | 0.996 | 0.32 |
| Post op. 1m | 12.40 ± 2.31 | 13.74 ± 3.17 | 1.7 | 0.12 |
| Post op. 3m | 15.06± 3.37 | 15.64 ± 3.41 | 0.605 | 0.52 |
| Post op. 6m | 15.36± 3.27 | 15.46 ± 2.78 | 0.116 | 0.9 |
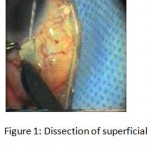 |
Figure 1: Dissection of superficial scleral flap.
|
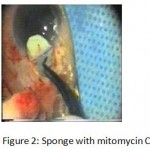 |
Figure 2: Sponge with mitomycin C.
|
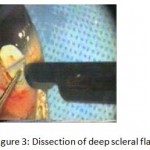 |
Figure 3: Dissection of deep scleral flap.
|
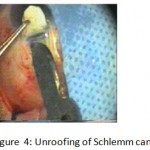 |
Figure 4: Unroofing of Schlemm canal.
|
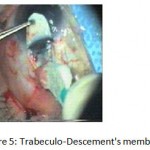 |
Figure 5: Trabeculo-Descement’s membrane.
|
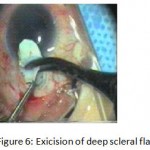 |
Figure 6: Exicision of deep scleral flap.
|
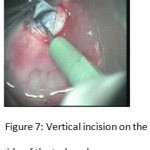 |
Figure 7: Vertical incision on the side of the trabeculum.
|
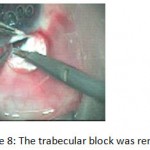 |
Figure 8: The trabecular block was removed.
|
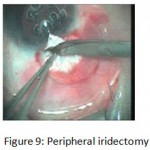 |
Figure 9: Peripheral iridectomy.
|
Results
Patients’ demographics: The range of patients’ age was 47 to 73 years with a mean of (56.8 ± 6.18) in group A and 48 to 71 years with a mean of (58.25 ± 8.46) in group B. There was no statistically significant difference (p=0.488) in age. In group A, 16 were females (64 %) while 9 were males (36%). In group B, 12 were females (48%) while 13were males (52%). There was no statistically significant difference (p=0.25) in sex. IOP was measured by applanation tonometer. Preoperative and postoperative intraocular pressure values are shown in table I. Success was defined as complete success if IOP < 21mmHg without anti glaucoma therapy or relative success if IOP < 21mmHg with topical one or two anti-glaucoma drugs. In group A, by the end of the first month, complete success was achieved in 96% of eyes (24 out of 25). By the end of the third month complete success was achieved in 84% of eyes (21 out of 25 eyes). By the end of the sixth month 80% (20 out of 25 eyes) in group A maintains complete success. In group B, by the end of the first month complete success was achieved in 100% of eyes (25 eyes). By the end of the third month complete success was achieved in 84% of eyes (21 out of 25eyes). By the end of the sixth month 84% (21 out of 25 eyes) in group B maintains complete success. In group A, by the end of the first month, one eye (4%) needs timolol maleate eye drops 0.5% to maintain IOP below 21mmHg, by the end of the third month, four eyes (16%) were controlled with adjunctive topical treatment. By the end of the sixth month, three cases (12%) required medical treatment and two cases (8%) required a trabeculectomy with MMC. Medical treatment used was combination of timolol maleate 0.5% and dorzolamide eye drops in two cases while the third case kept under betaxolol 0.5% eye drops. In group B, by the end of the first month, no adjunctive medical treatment was needed but by the end of the third month, four eyes (16%) needed timolol maleate 0.5% to maintain IOP below 21mmHg. By the end of the sixth month, three eyes (12%) still needed adjunctive topical treatment for IOP to be controlled; one was kept under combination of timolol maleate eye drops 0.5% and dorzolamide eye drops, the other two cases were controlled only with betaxolol 0.5%. The fourth case underwent another trabeculectomy with MMC in the superionasal quadrant. The total success rate, comprising complete and qualified success, attained at the end of the follow up period was 92% in group A (23 eyes), while it was 96% in group B (24 eyes). There was no significant difference between the two groups (p= 0.88) using X2 (Chi square test). At the end of the follow up period, the magnitude and percentage of pressure reduction were calculated in each group and it showed a statistically highly significant value in each group compared to the preoperative values. The reduction of IOP was slightly higher among patients in group A than in group B but was not statistically significant (as shown in table II).
Table 2: Postoperative IOP reduction.
| Group A | Group B | T test | P | |
| Magnitude | ||||
| MD | 15.32 | 15.26 | 0.03 | 0.97 |
| SD | ± 6.02 | ± 6.12 | ||
| Percentage | 0.07 | 0.93 | ||
| MD | 49.9 | 49.6 | ||
| SD | ± 15.8 | ± 11.44 |
Table 3: Comparison between the two groups concerning change in VA using t. test.
| VA | Group A | Group B | t-test | P |
| Mean ± SD | Mean ± SD | |||
| Pre op. | 0.048 ± 0.073 | 0.066 ± 0.079 | 0.83 | 0.37 |
| Post op. 1 w | 0.288 ± 0.166 | 0.297 ± 0.157 | 0.197 | 0.84 |
| 1m | 0.427 ± 0.217 | 0.508 ± 0.306 | 1.08 | 0.26 |
| 3m | 0.632 ± 0.301 | 0.649 ± 0.319 | 0.19 | 0.84 |
| 6m | 0.655 ± 0.306 | 0.662 ± 0.297 | 0.08 | 0.93 |
Preoperative and postoperative visual acuity (using the decimel equivalent of the ratio recorded on the Snellen acuity testing) is shown in table III. At the end of the six post operative month, BCVA rang was 1/60 to 6/6 with a mean of 0.655±0.306 in group A and 1/60 to 6/6 with a mean of 0.662±0.297 in group B without a statistically significant difference between the two groups (p= 0.93). At the end of follow up period, five patients (three in group A & two in group B) had mild (less than 6/60) improvement of best corrected visual acuity than the preoperative level due to advanced glaucomatous damage. Visual acuity results were not related to the surgery. No patients had deterioration of visual acuity level.
Intraoperative complications include visible perforation of the trabeculo-descement’s membrane which occurred during the dissection of the deep scleral flap in three eyes. In these three eyes (12%), we switched over to trabeculectomy procedure. These patients were excluded from the study, and replaced. Rupture of the posterior capsule with subsequent vitreous loss occurred in one case (4%) during Phaco-deep sclerectomy. Automated anterior vitrectomy was performed and posterior chamber IOL was implanted on the rim of the posterior capsule. The case did not show an elevation of IOP postoperatively. While during phaco-trabeculectomy, rupture of the posterior capsule and subsequent vitreous loss occurring in two cases (8%). Automated anterior vitrectomy was performed and posterior chamber IOL was implanted on the rim of the posterior capsule. None of these eyes showed elevation of the intraocular pressure postoperatively and both were controlled without medications.
In group A, one eye (4%) had postoperative hypotony (IOP less than 6 mmHg). It showed a slight gradual rise of IOP within the following few weeks. The final IOP in this case was 14 mmHg with complete success by the sixth month. Meanwhile in group B, three eyes (12%) had postoperative hypotony during the early postoperative days. All cases showed a slight gradual rise of IOP within the following few weeks, so that none of these cases showed persistent hypotony after 4 weeks postoperatively. The mean final IOP in these cases was 15.2 mmHg at the end of the follow up period. All cases of early postoperative hypotony had complete success by the sixth month. There were no cases of choroidal detachment in group A, however in group B, it occurred in one cases (4%) and was treated conservatively by frequent topical and systemic steroids. Improvement occurred in visual acuity and the choroidal detachment disappeared completely after two weeks. Postoperative hyphema was noticed in two cases (8%) in group B only. One patient had 2-mm hyphema& the other had less than 1mm hyphema. Both resolved spontaneously within 1-2 weeks without any effect on visual acuity or intraocular pressure.
Discussion
The management of patients with coexisting cataract and glaucoma is a common and challenging clinical problem. Coexisting cataract and glaucoma can be managed with one of three basic surgical approaches (1) cataract surgery first then glaucoma operation or anti-glaucoma medications, (2) glaucoma operation first then cataract operation, or (3) combined cataract extraction and glaucoma surgery, each of the three management strategies had its drawbacks.
Recent advances in phacoemulsification techniques and non penetrating glaucoma filtration surgeries have come together to revolutionize the surgical management of coexisting cataract and glaucoma and have made the combined procedure a more viable option than in the past. In this study we attempt to compare postoperative IOP after combined phacoemulsification and deep scelerectomy with mitomycin C (group A) versus combined phacoemulsification and trabeculectomy (group B). Our results were comparable to the results of Funnel CL et al6 who compared the outcomes of phacoemulsification combined with trabeculectomy (PT) or deep sclerectomy (PDS) with intraoperative mitomycin C (MMC) application in 97 eyes of 97 patients (59 PDS, 38 PT). In their study, no statistically significant difference was found in the IOP. In PDS group, at the end of the sixth post operative month, the mean IOP was 14.5mmHg. In PT group, at the end of the sixth post operative month, the mean IOP was 12mmHg. Mermoudet al7 compared 30 eyes that underwent phacotrabeculectomy (group I) and 30 eyes that underwent phaco deep sclerectomy (group II). They found no statistically significant difference between mean follow up IOP in both groups during 1.5 years follow up period. They reported complete tonometric success without any anti-glaucoma therapy in 64% in group I and in only 60% in group II. In the present study, complete success was achieved in 80% of cases (20 of 25 eyes) in group A and 84% (21 of 25 eyes) in group B by the end of the sixth month. The higher percentage of complete success in our study may be due to the relatively shorter follow up period (6 months) and the intraoperative application of MMC which efficacy was evaluated by Anand S and Anand N8. They studied 119 eyes (63 with and 56 without MMC augmentation) of 119 patients to evaluate the safety and efficacy of intraoperative mitomycin (MMC) augmentation of combined phacoemulsification and deep sclerectomy (PDS). The mean follow-up was 23 months. Two years after surgery, the probability of maintaining an IOP below 19 mmHg without glaucoma medications or needle revision was 76% in the PDS-MMC group and 62% in the PDS-no MMC group (P=0.02). They reported that MMC augmentation appears to increase the probability of achieving lower target intraocular pressures after combined PDS.
Funnel CL et al6 found no differences in the improvement of visual acuity between the two groups. They attributed cases of poor visual results to age related macular degeneration (one eye, PT group), epiretinal membrane (one eye, PT group), ischemic central retinal vein occlusion (one eye, PDS group).
In the present study, postoperative hypotony occurred in one eye (4%) in group A during the early postoperative days. It showed a slight gradual rise of IOP within the following few weeks. The final IOP in this case was 14 mmHg with complete success by the sixth month. Meanwhile in group B, three eyes (12%) had postoperative hypotony (IOP less than 6 mmHg) during the early postoperative days.
The mean final IOP in these cases was 15.2 mmHg at the end of the follow up period. All cases of early postoperative hypotony had complete success by the sixth month. Wedrich et al4 noted the occurrence of early post-operative hypotony as follows: hypotony less than 10 mmHg in 35% of cases, less than 5mmHg in 12% of cases (these cases were resolved completely during the first week), and 2% of cases showed persistent hypotony with the development of choroidal effusion. Mammalis et al9 reported that post-operative hypotony (6mmHg or less) is a sign of success of filtration surgery especially in the first week. García-Pérez JL et al10 studied the intraocular pressure (IOP) on the first day as a prognostic indicator in 70 eyes who had combined phacoemulsification-non penetrating deep sclerectomy. They used a split point of 9.0 mm Hg on the first postoperative day. They observed a greater success rate in patients with an IOP of 9 mmHg or less on the first postoperative day; these patients also had a significantly reduced need for glaucoma treatment. They recommended that an IOP of 9 mm Hg or less on the first postoperative day might serve as a positive prognostic indicator in combined phacoemulsification with deep sclerectomy.
In the present study, postoperative hypheama developed in two cases (8%) in group B only. Both resolved spontaneously within 2 weeks without any effect on visual acuity or intraocular pressure. . Mermoud et al7 reported incidence of postoperative hyphema in 6.7% in P-DS and 36.7% in P-T. Funnell CL et al6 reported incidence of 5% in P-DS and 5.3% in P-T
In Conclusion, P-DS with MMC does not exceed conventional PT in what concerns IOP reduction. However the complication rate is lower in P-DS group. Augmentation of PDS with MMC is safe with few serious complications related to its use and MMC augmentation appears to increase the probability of achieving lower target intraocular pressures after combined PDS.
Regardless of the limitations of our study, derived from relative small number of cases, short follow-up time, our results in what concern control of IOP, reduction of medication, recovery of vision and absence of severe complications lead us to further enthusiasm considering combined PDS with MMC in this type of patients. Further broader studies, with long term and improved follow-up will assist in further evaluating the efficacy of this combined surgical technique.
Acknowledgments
I wish to thank Prof. DrHosneia Mohamed Rageb, Professor of Community medicine, Faculty of Medicine, Zagazig University, who has been kind enough to spare no effort and no time to provide statistical consultation and assistance.
References
- Seah SKL, Jap A, Praia JA et al. Cataract surgery after trabeculectomy. Ophthalmic Surg Lasers, 27: 587-94 (1996).
- Palmberg P: Combined cataract and glaucoma surgery with Mitomycin-C. In: New developments in glaucoma, Ophthalmology Clinics of North America, Stamper RL, Lee DA (eds). U.S.A: W.B Saunders Company. 8(2): 365-381 (1995).
- Munden PM, Alward WLM. Combined phacoemulsification, posterior chamber intraocular lens implantation, and trabeculectomy with mitomycin-C. Am J Ophthalmol; 119: 20-29 (1995).
- Wedrich A, Menapace R, Radax U, Papapanes P. Long-term results of combined trabeculectomy and small incision cataract surgery. J Cataract Refract Surg., 21(1): 49-54 (1995).
- Gianoli F, Schnyder C. Bovcy E, Mermoud A. Combined surgery for cataract and glaucoma: phacoemulsification and deep sclerectomy compared with phaco-emulsification and trabeculectomy. J Cataract Refract Surg 25: 340-46 (1999) .
- Funnell CL, Clowes M, Anand N. Combined cataract and glaucoma surgery with mitomycin C: phacoemulsification-trabeculectomy compared to phacoemulsification-deep sclerectomy. Br J Ophthalmol., 89(6): 694-8 (2005).
- Mermoud A, Fabrice G, Corinne C and Schnyder R: Combined surgery for cataract and glaucoma: phacoemulsification and deep sclerectomy compared with phacoemulsification and trabeculectomy. J cataract Refract Surg, 25(3): 340-346 (1999).
- Anand S and Anand N. Combined phacoemulsification and deep sclerectomy (PDS) with intraoperative mitomycin C (MMC) augmentation. Eye (Lond)., 22(8): 1040-9 (2008).
- Mammalis N, Loner S, Rand AN, and Crandoll AS: Combined phacoemulsification, intraocular lens implantation and trabeculectomy. J. Cataract Refract, Surg., 22: 467-473 (1996).
- García-Pérez JL, Rebolleda G, Muñoz-Negrete FJ. Intraocular pressure on the first postoperative day as a prognostic indicator in phacoemulsification combined with deep sclerectomy. J Cataract Refract Surg. 34(8): 1374-8 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.





