Manuscript accepted on :
Published online on: --
Functional Properties of Amoora Rohituka Seed Protein Isolates
Moumita Gupta, P. Kundu and S. Laskar*
Natural Products Laboratory, Department of Chemistry, The University of Burdwan, Burdwan - 713 104 India.
Corresponding Author E-mail:slaskar01@yahoo.co.in
ABSTRACT: Functional properties of a total protein isolate (TPI) prepared from the seeds of Amoora rohituka were determined. The effects of pH on some of these functional properties were also investigated. The protein content of the total seed protein isolate was found to be 65.09%. The minimal protein solubility was observed at pH 3 and the maximum was at pH 12. Water- and oil-holding capacities of the total seed protein isolates were 2.01g.g-1 and 5.88g.g-1. The emulsifying activity and emulsion stability, as well as foaming capacity and foam stability, were greatly affected by pH levels. Lower values were observed at slightly acidic pH (pH 4.0). Total protein isolates (TPI) was highly viscous which depend on concentration and pH levels. The least gelation concentration of seed protein isolate was found to be 16% when the proteins were dissolved in distilled water.
KEYWORDS: Amoora rohituka; seed protein isolate; nitrogen solubility; functional properties.
| Copy the following to cite this article: Gupta M, Kundu P, Laskar S. Functional Properties of Amoora Rohituka Seed Protein Isolates. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Gupta M, Kundu P, Laskar S. Functional Properties of Amoora Rohituka Seed Protein Isolates. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9557 |
Introduction
Studies of plant protein, as a non-conventional protein source, have been on the increase, due to the new challenge of providing adequate protein for an expanding world population. Also, exploiting such resources can boost economic development, especially in the developing countries, where protein intake is less than that required1. Due to more expensive and relatively difficult to acquire of animal proteins, such as milk, meat, eggs etc, there has been a constant search of non-conventional plant seeds as new protein sources for use as both functional food ingredients and nutritional supplements2. Consequently, during the last four decades the food industry has placed special attention on the utilization of seed protein. Aphanamixis polystachya syn. Amoora rohituka (Pittaraj), which belongs to the family Meliaceae has been chosen for present study for the aforesaid purpose. This plant is widely distributed in many parts of India, especially in Uttarpradesh, West Bengal, Assam, Sikim, Chota Nagpur region, and Western ghats. This plant has several folk medicinal uses in Indian villages. Seeds of this plant may be marked as non traditional class and practically no proper utilization of them, have been chosen for the present study. No proper attempts have been made to isolate the protein of this seeds, even though it contains appreciable amounts of nitrogen and storage protein. Our intension is to find out new proteins for human and animal consumption from such non-conventional seed materials. A chemical characterization on Amoora rohituka seed protein has already been done by the authors and favorable results have been found in connection with this objective3. Therefore, these plant proteins to be useful and successful in food application, they should ideally possess several desirable characteristics, referred to as functional properties. With the improvement of the functional properties of seed protein concentrate through processing these seed proteins may be used in manufactured foods and texturized products for human consumption4. Proteins are unstable to heating, organic solvents and proteolytic attack, so they have limited use in food industries. If proteins could be converted into stable forms, their application would be greatly broadened. Attempts have been carried out to modify non conventional plant seed protein to improve their physical functionality i.e solubility, viscosity, gelation, emulsifying and foaming properties5. Factors, such as pH and salts, affect physicochemical properties and interaction between proteins and in turn alter functional properties6-8. The objective of the present study was to investigate the functional properties (e.g., solubility, water absorption, oil absorption, foaming, emulsifying, viscosity, gelation etc.) of the seed protein isolates of A. rohituka and to predict their applications as alternative food ingredient.
Material and Methods
Materials
Mature fruits of A. rohituka were collected from Burdwan Forest Department, Burdwan, West Bengal, India, and authenticated by Professor A. Mukherjee, Department of Botany, Burdwan University, West Bengal, India. A voucher specimen Burdwan, Moumita 245 has been deposited at the herbarium of the Botany Department, Burdwan University, Burdwan, bearing acronym BURD. Fruits were de-coated, and collected seeds were then air dried. The air dried seeds were then used for protein extraction. All reagents used in the investigation were of analytical grade. Corn oil was obtained from National Chemicals, Vadodara, India.
Preparation of protein isolates
The air dried seeds were crushed to powder by a hand crusher and the seed flour (550 gm) was then defatted by extracting with 3 L petroleum ether (40-60 °C) at room temperature (20 ºC) for 21 days. It was then washed thrice with CHCl3: MeOH (3:1) and then air dried. The defatted flour was extracted by stirring with distilled water using a flour to solvent ratio 1:20 for 45 min and the pH was adjusted to 7.0 by 0.5M NaOH/0.5M HCl. The suspension obtained was first filtered through cotton and finally centrifuged at 10,000g for 20 min. The pH of the supernatant was lowered to pH of minimum solubility (3.0) of the seed protein by 10% trichloroacetic acid. This pH is probably near the isoelectric point of the protein. The precipitate thus formed was recovered by centrifuge at 10,000g for 20 min. The precipitate was redissolved with deionised distilled water of pH 7.0, dialyzed against distilled water for 72 h at 4 °C, freeze dried and finally stored in refrigerator at 4 ºC for further use.
Proximate analysis
The ash and moisture contents of the protein isolates were determined according to AOAC procedures9. Nitrogen content of the protein isolate was determined by the micro-Kjeldahl technique, following the method of the A.O.A.C9. Percentage of the nitrogen was converted to crude protein by multiplying with 6.25. All the results were place in Table 1.
Table 1: Proximate chemical analysis of the seed protein isolate of A. rohituka.
| Parameter | Valuea |
| Moisture (%) |
9.83 ± 0.15 |
| Ash (%) | 1.79 ± 0.05 |
| Nitrogen (%) | 10.41 ± 0.06 |
| Protein (%) | 65.09 ± 0.39 |
a Values are mean ± S.D., n= 3
Nitrogen solubility
Nitrogen solubility was determined using the method of Were et al.10 with slight modification. Sample (125 mg) was dispersed in 25 ml of distilled water and the solution pH was adjusted to 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 using either 0.5 M NaOH or 0.5 M HCl. The dispersion were agitated for 45 min with the aid of magnetic stirrer and then centrifuged at 10000 g for 15 min. Nitrogen content of the supernatant after freeze-drying, was determined by Kjeldahl method9. Triplicate determinations were carried out, and the solubility profile was obtained by plotting averages of protein solubility (%) against pH. The percent soluble protein was calculated as follows:

Water- and oil-holding capacity
The method of Carcea Bencini11 was used with slight modification. One gram of protein sample in each case was stirred in 10 ml of distilled water of pH 7.0 or corn oil for 1 min. the samples were then allowed to stand separately at room temperature (20 ºC) for 30 min and centrifuged at 5000 g for 15 min. The volume of the supernatant was noted in a graduated cylinder. The water-holding capacity is expressed as the number of grams of water held by 1 g protein sample. The oil-holding capacity is expressed as the number of grams of oil held by 1 g protein sample. Density of corn oil was found to be 0.92 g.ml-1. Values of water and oil-holding capacities are placed in Table 2.
Table 2: Water- and oil-holding capacities of A. rohituka seed protein isolate.
| Parameter
|
Valuea |
| Water Holding Capacity (g/g)
|
2.01 ± 0.07 |
| Oil Holding Capacity (g/g)
|
5.88 ± 0.04 |
a Values are mean ± S.D., n= 3
Emulsifying activity (EA) and Emulsion stability (ES)
Emulsions were prepared according to the method of Sathe et al.12 with following modifications. Emulsion properties were determined as a function of pH (2, 4, 6, 8 and 10). 5 ml of 2% seed protein suspension at different pH (2 to 10) was homogenized at high speed for 30 s, and 5 ml of corn oil was then added and homogenized for another 1 min. The emulsions were centrifuged at 3000 g for 5 min and the volume of the emulsion left was measured.
Emulsion activity (EA) was calculated as follows:
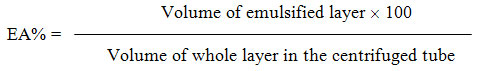
To determine the emulsion stability (ES), emulsions prepared by the above way were heated at 80 °C for 30 min, and then cooled to room temperature and centrifuged at 3000 g for 5 min. Emulsion stability (ES) was calculated as follows:

Foaming properties
Foam capacity and stability at different pH levels were determined according to the method of Venktesh & Prakash4 with slight modification. 1.5 gm protein isolate in 100 ml distilled water at different pH, of different concentrations were separately dispersed with the use of an ultrasonic vibrator (Model No. Transsonic T660/H, Elma, Germany). Then it was whipped in an electrical stirrer for 5 min at medium speed and was poured in a 250 ml graduated cylinder. The volume of foam at 30 s was calculated, and the volume increase is expressed as percent foam capacity. Foam stability was determined by measuring the decrease in volume of foam as function of time up to a period of 120 min.

Viscosity of seed protein
Relative viscosity of different protein concentration or pH levels were determined according to the method Hazra & Laskar13. The sample was dispersed in distilled water with the aid of the ultrasonic vibrator at room temperature (20 ºC) prior to viscosity measurement. Each sample was prepared at concentration of 1, 2, 4, 8 and 10% (w/v) and 2% (w/v) solution of different pH levels (2, 4, 6, 8, and 10). Relative viscosity (relative to water) was measured at room temperature (20 °C) employing an Ostwald type viscometer. All the measurements were done in triplicate and means (relative to distilled water) are reported (Table 3).
Table 3: Effect of protein concentration and pH on viscosities of A. rohituka seed protein isolates at room temperature (20°C).
| Protein Concentration (g/100 ml) | Viscosity (cp)a | pH | Viscosity (cp)a | |
| 1 | 0.94 ± 0.05 | 2 | 0.72 ± 0.05 | |
| 2 | 1.08 ± 0.07 | 4 | 1.03 ± 0.08 | |
| 4 | 1.83 ± 0.11 | 6 | 1.19 ± 0.03 | |
| 6 | 2.24 ± 0.15 | 8 | 1.24 ± 0.07 | |
| 8 | 3.88 ± 0.21 | 10 | 1.87 ± 0.04 | |
| 10 | 5.44 ± 0.33 | – | – | |
a Values are mean ± S.D., n= 3
Gelation capacity
Gelation capacity of this seed protein was employed according to the method of Coffmann & Garcia14 with slight modification. Appropriate sample suspension of 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20% (w/v) were prepared in 5ml distilled water with the use of ultrasonic vibrator. The test tubes containing these suspensions were then heated for 1 h in a boiling water bath, followed by rapid cooling under running cold tap water. The test tubes were then further cooled for 2 h at 4 ºC. The least gelation concentration was determined as that concentration when the samples from the inverted test tubes did not fall down or slip (Table 4).
Table 4: Gelation capacity (GC) of A. rohituka seed protein isolate in water at different concentrationsa .
| Protein concentrates (g/100ml) |
GC in H2O |
| 4 | – |
| 6 | – |
| 8 | – |
| 10 | – |
| 12 | – |
| 14 | – |
| 16 | + |
| 18 | + |
| 20 | + |
a Results are means of triplicate samples.
Results and Discussion
Proximate analysis
The moisture, ash, nitrogen and protein content of the protein isolates are shown in Table 1. The protein content of the Amoora rohituka protein isolate was lower than compared to that high protein content seed such as Chinese indigenous legume seed and soybean protein (79.6%, 78.0%, 85.0%, 78.7%)15. But it is comparable with other two legume seeds (71.0%, 73.0%)16; tomato seed (71.0%)17; and amaranth seed protein (67.0%)18 and consequently its protein content is considerably high.
Nitrogen solubility
The nitrogen solubility profile (Fig. 1) of the protein isolate indicates that the minimum nitrogen solubility was observed at a pH of about 3. On either side of pH 3, there was a sharp increase in the nitrogen solubility of the protein isolate. At pH 2, about 50.83% of the nitrogen was soluble, and about 97.53% of the nitrogen was soluble at pH 11. So the nitrogen solubility studies showed good solubility in both acidic and alkaline pH regions, which is an important characteristic for food formulation19. Seena & Sridhar20 reported that, at highly acidic and alkaline pH, the protein acquires net positive and negative charges, respectively, which favor the repulsion of molecules and thereby increase the solubility of the protein.
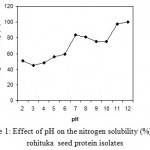 |
Figure 1: Effect of pH on the nitrogen solubility (%) of A. rohituka seed protein isolates.
|
Water- and oil-holding capacity
Water-holding capacity (WHC) and oil-holding capacity (OHC) of the protein isolates were 2.01g.g-1 and 5.88g.g-1 respectively (Table 2). Water holding capacity (WHC) of the seed protein isolate in the present investigation was higher than that of lupin seed protein concentrate (1.37 g.g-1)21 and bakul (Mimusops elengi L.) seed protein concentrate (1.70g.g-1)13 but was lower than Soybean protein concentrate (3.46 g.g-1) and three legume seed protein concentrate P. angularis, P. calcaratus, and D. lablab (5.05, 5.25, 5.08g.g-1 respectively) reported by Chau et al.15. Different proteins have different WHC due to different protein conformations and the variations in number and nature of the water binding sites on protein molecules22. The WHC is a critical property of protein in viscous food e.g. soups, dough, custards and baked products, because these are supposed to imbibe water without dissolution of protein, thereby providing body thickening and viscosity20, 23.
Oil-holding capacity (OHC) of A. rohituka seed protein isolate was higher than that of soybean protein concentrate (3.06 g.g-1) and three legume seed protein concentrates (4.38, 4.71 and 4.77 g.g-1 respectively)15. The higher OHC might be attributed to a higher level of non polar side chains in their molecules. Such high OHC make the protein potentially useful in structural interactions in food, especially in flavor retention, improvement of palatability and extension of shelf life in meat products through reduction of moisture and fat loss.
Emulsifying activity (EA) and Emulsion stability (ES)
The emulsifying activity (EA) and emulsion stability (ES) are greatly affected by different pHs. Amoora rohituka seed protein isolate had a minimum emulsifying activity at pH 4 and emulsifying activities are increasing on either side of pH 4 (Fig.2). This protein isolate exhibited good EA values (53.33-59.87%) at different pH levels. with values similar to those of soybean protein isolate (54-58%)15. Dependence of emulsion activity on pH was expected, as it is known that emulsion activity of a total protein dependence on the hydrophilic-lipophilic balance, which is affected by pH21.
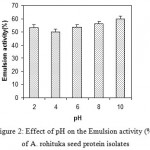 |
Figure 2: Effect of pH on the Emulsion activity (%) of A. rohituka seed protein isolates.
|
Like emulsion activity (EA), emulsion stability (ES) of this protein isolate was higher at both acidic and alkaline pH (Fig.3). Hung & Zayas24 suggested that various factor including pH, droplet size, net charge, interfacial tension, viscosity and protein conformation could affect the values of ES. In this case, the high ES (in different pH) which involved during heating of protein at 80 ºC for 30 min might be attributed to the dissociation of some protein molecule, and the resulting solutions formed had more hydrophobic groups, which interacted more strong with the lipid phase.
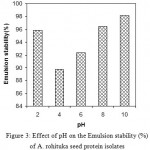 |
Figure 3: Effect of pH on the Emulsion stability (%) of A. rohituka seed protein isolates.
|
Foaming properties
The results of foaming capacity (FC) of A.rohituka seed protein isolate indicated that its FC was pH dependent (Fig.4). The lowest FC (25%) was obtained at pH 4 (probable iso-electric point of protein). FC significantly increased, especially at pH 8 and 10 reaching 50% and 58% respectively. ). The higher FC at pH 8 and 10 was due to the increased net charges on the protein, which weakened the hydrophobic interactions but increased the flexibility of the protein. This allowed the protein to diffuse more rapidly to the air-water interface to encapsulate air particle and then enhance foam formation25.
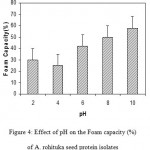 |
Figure 4: Effect of pH on the Foam capacity (%) of A. rohituka seed protein isolates.
|
The effect of pH on foam stability (FS) as a function of time is shown in Fig. 5. It showed that the FS decreased with time. From pH 2 to 10, the foam stability decreased with time. Such pH and time dependent of foaming stability was also reported for soybean and sunflower protein26.
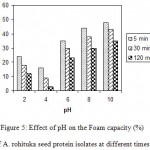 |
Figure 5: Effect of pH on the Foam capacity (%) of A. rohituka seed protein isolates at different times
|
Viscosity of seed protein
The results of viscosity of A. rohituka seed protein isolate at different concentration at pH 7 and also at different pH levels at room temperature (26 °C) are presented in Table 3. A significant increase in the viscosity with increasing protein concentration was observed. Concentration dependence of viscosity was also reported for sunflower protein concentrate27 and soybean protein isolates15. At room temperature, the viscosity of protein solution (2%) showed marked increase in viscosity at both acid and alkaline pHs. Results of the present study indicate that the viscosity of the protein depends on the pH, which is likely to affect the conformational characteristics of protein, since viscosity is conformation dependent19.
Gelation capacity
Gelation capacity of this seed protein isolate is shown in Table 4. The least gelation concentration for A. rohituka seed protein was found to be 16% (w/v), when it was dissolved in distilled water. According to Schmidt28, considerably higher protein concentrate is usually required for the gelation of globular proteins. So it can be concluded that gelation is not only a function of protein quantity but seems also to be related to the type of protein as well as to the non protein components and protein solubility. A similar conclusion was reached by Sathe & Salunkhe in their study29.
Conclusion
The functional properties of A. rohituka seed protein were evaluated to asses their effective use in food systems. It was found that A. rohituka seed protein showed high solubility as the solution approached neutrality and alkaline pH. The oil-holding capacity (OHC) and water-holding capacity (WHC) are also higher than any other standard seed protein, which is very important for the preparation of sausages, doughnuts (requiring high OHC) in food industries. The high emulsifying properties and foaming properties have pointed an important role in food systems, such as salad dressing, comminuted meat (requiring high EA & ES), ice-cream, cake batters, whipped toppings, chiffon deserts, sponge cakes (requiring high FS). The high emulsion stability indicates its usefulness in seasoning, manufacturing especially in products that require heating, as the protein-lipid interaction is favored by the temperature increase. The better viscosity values at acid and alkaline pH is also important in food industry. Gelation capacity is also an important function in food formulations. Due to these properties, the seed protein isolate of Amoora rohituka is very attractive as functional ingredients in food systems but sensory and texture analysis of the product would be necessary.
Acknowledgements
Authors are grateful to Council of Scientific and Industrial Research, New Delhi, India for financial assistances to carry out such research work.
References
- Satterle, L.D. Proteins for use in foods. Food Tech., 35: 53-70 (1981).
- Onweluzo, J.C.; Obanu, Z.A.; Onuoha, K.C. Functional properties of some lesser known tropical legumes. Food Sci. Tech., 31: 302-306 (1994).
- Gupta, M.; Sarkar, K.; Baral, R.N.; Laskar, S. Some chemical investigations of Amoora rohituka seed proteins. Food Chem., 119: 1057-1062 (2010).
- Venktesh, A.; Prakash, V. Functional properties of total proteins of sunflower (Helianthus annuus) seed- effect of physical and chemical treatments. J. Agric. Food Chem., 41: 18-23 (1993).
- Sakamoto, H.; Kumazawa, Y.; Motoki, M. Strength of protein gels prepared with microbial transglutminase as related to reaction conditions. Food Sci., 59: 866-871 (1994).
- Kinsella, J.E. Functional properties of soy proteins. Am. Oil Chem. Soc., 56: 242-258 (1979).
- Mwasaru, M.A.; Muhammad, K.; Bakar, J.; Cheman, Y.B. Influence of altered solvent environment on the functionality of pigeon pea (Cajanas cajan) and cowpea (Vegna ungviculata) protein isolates. Food Chem., 71: 157-165 (2000).
- Philips, L.G.; Yang, S.T.; Kinsella, J.E. Neutral salts effect on stability of whey protein isolate foams. Food Sci., 56: 588-589 (1991).
- Official Methods of Analysis; 15th ed., Association of Official Analytical Chemists: Washington, DC (1990).
- Were, L.; Hettiarachchy, L.; Kalapathy, U. Modified soy proteins with improved foaming and water hydration properties. Food Sci., 62: 821-824 (1997).
- Carcea Bencini, M. Functional properties of drum dried chickpea (Cicer arietinum) flours. J. Food Sci., 51: 1518-1521 (1986).
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of black gram (Phaseolus mungo) proteins. Lebensm-Wiss. Technol., 16: 69-72 (1983).
- Hazra, K.M.; Laskar, S. Functional properties of protein concentrates from Mimusops elengi seed. Acta Alimentaria., 34(4): 473-482 (2005).
- Coffman, C.; Garcia, V.V. Functional properties and amino acid content of a protein isolate from mung bean flour. Food Technol., 12: 473-484 (1977).
- Chau, C.F.; Cheung, P.C.K.; Wong, Y.S. Functional properties of protein isolates from three Chinese indigenous legume Seeds. Agric. Food Chem., 45(7): 2500-2503 (1997).
- Guerrero, L.C.; Flores, V.P.; Ancona, D.B.; Ortiz, G.D. Functional properties of flours and protein isolates from Phaseolus lunatus and Canavalia ensiformis J. Agric. Food Chem., 50: 584-591 (2002).
- Liadakis, G.N.; Tzia, C.; Oreopoulou, V.; Thomopoulos, C.D. Isolation of tomato seed meal proteins with salt solutions. Food Sci., 63: 450-453 (1998).
- Fidantsi, A.; Doxastakis, G. Emulsifying and foaming properties of amaranth seed protein isolates. Colloids and Surfaces B: Biointer., 21: 119-124 (2001).
- Idouraine, A.; Yensen, S.B.; Weber, C.W. Tepary bean flour, albumin and globulin fractions functional properties compared with soy protein isolate. Food Sci., 56: 1316-1318 (1991).
- Seena, S.; Sridhar, K.R. Physiochemical, functional and cooking properties of under explored legumes Canavalia of the southwest coast of India. Food Res. Int., 38: 803-814 (2005).
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrate. Food Sci., 47: 491-497 (1982).
- Chou, D.H.; Morr, C.V. Protein-water interactions and functional properties. Am. Oil Chem. Soc., 56: 53-62 (1979).
- Adeyeye, E.I.; Oshodi, A.A.; Lpinmoroti, K.O. Functional properties of some varieties of African yam bean (Sphenostylis stenocarpa) flour II. J. Food Sci. Nutr., 45: 115-126 (1994).
- Hung, S.C.; Zayas, J.F. Emulsifying capacity and emulsion stability of milk protein and corn germ protein flour. Food Sci., 56: 1216-1218 (1991).
- Aluko, R.E.; Yada, R.Y. Structure-function relationships of cowpea (Vigna unguiculata) globulin isolate: influence of pH and NaCl on physiochemical and functional properties. Food Chem., 53: 259-265 (1995).
- Lin, M.J.Y.; Humbert, E.S.; Sosulski F.W. Certain functional properties of sunflower meal products. Food Sci., 39: 368-370 (1974).
- Flleming, S.E.; Sosulski, F.W.; Kilara, A.; Humbert, E.S. Viscosity and water absorption characteristics of slurries of sunflower and soybean flours, concentrates and isolates. J. Food Sci., 39: 188-191 (1974).
- Schmidt, R.H. Gelation and Coagulation. In Protein functionality in Foods; Cherry, J. P., Editor; American Chemical society, ACS Symposium series 147: Washington, DC, 131 (1981).
- Sathe, S.K.; Salunkhe, D.K. Functional properties of the great northern bean (Phaseolus vulgaris) proteins: emulsion, foaming, viscosity and gelation properties. J. Food Sci., 46: 71-81 (1981).

This work is licensed under a Creative Commons Attribution 4.0 International License.





