Manuscript accepted on : March 09, 2010
Published online on: --
Antimicrobial Efficacy of Boerhaavia Diffusa Against Some Human Pathogenic Bacteria and Fungi
Shilpa Wagh* and N. N. Vidhale
Shri Shivaji Science College, Shivaji Nagar, Morshi Road, Amravati - 444 602 India. Corresponding Author E-mail: deshchin07@rediffmail.com
ABSTRACT: The occurrence of side effects after a long term use of synthetic drugs is always feared during the treatment of chronic diseases. Such possibilities are experienced to be of negligible extent in the case of herbal drugs and thus are more efficient to treat infectious diseases than synthetic antibiotics. Therefore it is necessary to evaluate, in a scientific base, the potential use of natural medicine for the treatment of infectious diseases. In the present investigation decoction of roots of Boerhaavia diffusa was evaluated for its antimicrobial potential against nine different human pathogenic bacteria and five fungi by agar well diffusion method. In action Boerhaavia diffusa is antihelmintic, aphrodisiac, cardiac-stimulant, diaphoretic, diuretic, emetic, expectorant, febrifuge and laxative, ophthalmic, stomachic in jaundice, strangury and tonic. It is useful in leucorrhoea, inflammation, asthma, constipation, cough and cold, dropsical swellings, eye and heart troubles, jaundice, tertial fever and gonorrhoea. Boerhaavia diffusa was found active against almost all Gram-negative bacteria tested including Pseudomonas aeruginosa, Escherichia coli, Salmonella typhimurium, Salmonella typhi, Enterobacter aerogenes and Klebsiella pneumoniae, except Proteus vulgaris and therefore can serve as a broad spectrum antibacterial agent against Gram-negative bacteria. The only Gram-positive bacteria sensitive to this plant decoction was Enterococcus faecalis. In case of fungi only Candida glabrata was found highly sensitive. The results indicate that Boerhaavia diffusa has broad spectrum antimicrobial potential against antibiotic resistant microorganisms.
KEYWORDS: Antimicrobial; Ayurveda; Boerhaavia diffusa; decoction; herbal.
Download this article as:| Copy the following to cite this article: Wagh S, Vidhale N. N. Antimicrobial Efficacy of Boerhaavia Diffusa Against Some Human Pathogenic Bacteria and Fungi. Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Wagh S, Vidhale N. N. Antimicrobial Efficacy of Boerhaavia Diffusa Against Some Human Pathogenic Bacteria and Fungi. Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9506 |
Introduction
The revival of interest in natural drugs especially those derived from plants and animals, started in the last decades mainly because of the widespread belief that natural medicines are healthier and safer than synthetic ones. This is because herbal medicine has a type of chemical balance, also the plant has the complete knowledge of the know how of its own ecosystem therefore if the medicinal property gets changed to some extent due to some reasons the plant has its own defense mechanism so it will try to maintain all its important characteristics. Also the emergence of resistance to conventional antimicrobials is a serious problem that physicians face. This necessitates constant development of newer agents, which can inhibit the growth of or kill resistant organisms. Therefore the study attempts to portray the potential role of Ayurveda. The objectives of the in vitro study are to validate efficacy of decoction of roots of Boerhaavia diffusa as an antimicrobial agent. Drugs prepared according to the Ayurvedic methods are often found to be simply marvelous. And that is why the extraction method for the antimicrobial study is according to the Ayurvedic pharmacognosy, ‘Bhaishjyakalpana’ (Mishra 1989). The method of extraction of active ingredients used here is decoction method using water as a solvent. Boerhaavia diffusa is also known as Spreading hogweed, pigweed (English), Punarnava, sophaghni (Sanskrit), khaparkhutti (Korku). In action it is antihelmintic, aphrodisiac, cardiac-stimulant, diaphoretic, diuretic, emetic, expectorant, febrifuge and laxative, ophthalmic, stomachic in jaundice, strangury and tonic. It is useful in leucorrhoea, inflammation, asthma, constipation, cough and cold, dropsical swellings, eye and heart troubles, jaundice, tertial fever and gonorrhoea (Bhattacharjee 1998 and Khare 2007). Korku tribes of Amravati district of Vidarbha region in Maharashtra also use this plant as a blood purifier for jaundice and urinary disorders. Punarnavine, xanthone, arbinofuranoside a flavonoid are some of the chemical constituents found in Boerhaavia diffusa (Khare 2007).
Materials and Methods
Preparation of decoction
The roots of Berberis aristata was collected from Nagarjun Medicinal Plants Garden, (P.K.V.), Akola (MS). The roots was dried in shade and powdered. 25 gm of this powder was soaked in thirty-two parts (800ml) of potable drinking water in a glass beaker and left this for overnight. The next day decoction as a general rule of Ayurveda was prepared by boiling in the glass beaker in a water bath over a slow fire till it reduces to one-fourth (200 ml) and the decoction was then strained through cloth first (Mishra 1989) and then through Whatmann filter paper no.1 two times to remove suspended particles. Thus the final concentration of decoction was found to be 125 mg/ml of water. The actual concentration of active principle is being represented by the concentration of powder in water.
Test microorganisms
Some of the Gram-positive and Gram-negative bacteria, yeast and fungi responsible for gastrointestinal diseases, respiratory tract diseases, urinary tract infections, enteric and other type of fever, septicemia, pyogenic infections and wound infections, infections of ear and eye, skin diseases etc. were selected for this study. They are:
Gram-negative bacteria
Escherichia coli ATCC 10536, Salmonella typhimurium ATCC 23564, Salmonella typhi ATCC 531, Klebsiella pneumoniae MTCC 109, Enterobacter aerogenes ATCC 13048, Proteus vulgaris NCIM 2027 and Pseudomonas aeruginosa ATCC 27853.
Gram-positive bacteria
Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 29212.
Fungi
Aspergillus flavus MTCC 277, Candida albicans MTCC 3017, Candida tropicalis MTCC 184, Candida glabrata MTCC 3019, Trychophyton rubrum MTCC 3272, Microsporum gypseum MTCC 4479 and Epidermophyton floccosum MTCC 613.
Preparation of inoculums
All microorganisms were obtained from NCIM (National Collection of Industrial Microorganisms) Pune, India and IMTECH (Institute of Microbial Technology) Chandigarh, India. Four to five colonies from pure growth of each test organism were transferred to 10 ml of Nutrient broth, MRS broth and Sabouraud’s dextrose broth depending upon the microorganisms and then incubated at 37 °C for 18-24 hours for bacteria and at room temperature (25-30°C) for different time intervals, according to the fungus.
Screening for antimicrobial activity
The antibacterial and antifungal activity was determined by using agar well diffusion method (Saeed et, al. 2007). The media used for antibacterial study was Nutrient Agar Hi-Media (M 244) with composition of Peptone (10 gm/lit), Beef extract (10 gm/lit), NaCl (5 gm/lit) and pH was adjusted to 7.4 + 0.2 (at 25 0C). Nutrient agar plates were inoculated with eighteen hour old broth culture of each test bacterium. The media used for antifungal activity was Sabouraud’s Dextrose Agar Hi-Media (M 033) with composition of Mycological peptone (10 gm/lit), Dextrose (40 gm/lit), and pH was adjusted to 5.6 + 0.2 (at 25 0C). Sabouraud’s Dextrose agar plates were inoculated with the spore suspension of the each test fungus. The inoculum (0.1 ml) was spread evenly over the plate with sterile glass spreader. A sterile cork borer of standard 8 mm diameter was used to cut the well on agar surface and 500 ul of the decoction was poured in the well. The control was kept using sterile distilled water. Three replica plates were prepared for each microorganism. The plates were incubated at 37 0C for 24 hours for bacteria and at room temperature (25-30 °C) for different time intervals according to the fungus. The results were observed and the zones of inhibition (ZOI) were measured from the three replica plates and the mean was recorded. The minimum inhibition concentration (MIC) was also determined by making the dilutions of the decoction as 75% (93.75 mg/ml), 50% (62.5 mg/ml), 25% (31.25 mg/ml), 10% (12.5 mg/ml) and 5% (6.25mg/ml). MIC was recorded as the lowest concentration of test sample that prevented the growth of the test bacteria and fungi. The results of in vitro antibacterial and antifungal activities were analyzed statistically and are presented in terms of mean zone of inhibition standard deviation.
Results and Discussion
The results of antimicrobial activities decoction made from roots of Boerhaavia diffusa are shown in Table 1. Among the tested bacteria Pseudomonas aeruginosa was found most sensitive. The diameter of zone of inhibition (ZOI) was 22 mm at 125 mg/ml concentration of the decoction and the minimum inhibition concentration (MIC) was found to be 31.25 mg/ml. Among the other tested Gram-negative bacteria, Escherichia coli ATCC 10536, Salmonella typhimurium ATCC 23564, Salmonella typhi ATCC531, Klebsiella pneumoniae MTCC 109 and Enterobacter aerogenes ATCC 13048 have shown good antibacterial activity with ZOI 12 mm, 17 mm, 14 mm, 14 mm and 18 mm at 125 mg/ml concentration respectively and MIC for all was found to be 125 mg/ml. Proteus vulgaris NCIM 2027 was found to be resistant to the decoction. Among the Gram-positive bacteria only Enterococcus faecalis has exhibited antibacterial activity with ZOI 17 mm at 125 mg/ml and the minimum inhibition concentration was the same (125 mg/ml). While Bacillus subtilis ATCC 6633 and Staphylococcus aureus ATCC 25923 were found to be resistant. In case of fungi only Candida glabrata MTCC 3019 has shown antiyeast activity with ZOI 45 mm at 125 mg/ml concentration of the decoction and the MIC was found to be 12.5 mg/ml. All the other fungi tested have shown resistance to the decoction. Figure 1 shows variation in sensitivity of the susceptible microorganisms.
Table 1: Antimicrobial activity of decoction of Boerhaavia diffusa (Punarnava).
| Sr. No. | Name of microorganisms | Zone of inhibition (ZOI) in millimeter at different concentrations of decoction. | Mean
(ZOI) standard deviation |
|||||
| 125 mg/ml | 93.75
mg/ml |
62.5
Mg/ml |
31.25
mg/ml |
12.5
mg/ml |
6.25
mg/ml |
|||
| 1 | Escherichia coli
ATCC 10536 |
12mm* | —– | —– | —– | —– | —– | 120.82 |
| 2 | Salmonella typhimurium
ATCC 23564 |
17 mm* | —– | —– | —– | —–
|
—– | 171.41 |
| 3 | Salmonella typhi
ATCC 531 |
14 mm* | —– | —– | —– | —– | —– | 141.63 |
| 4 | Klebsiella pneumoniae
MTCC 109 |
14 mm* | —– | —– | —– | —– | —– | 140.82 |
| 5 | Enterobacter aerogenes
ATCC 13048 |
18 mm* | —– | —– | —– | —– | —– | 180.82 |
| 6 | Proteus vulgaris
NCIM 2027 |
—– | —– | —– | —– | —– | —– | |
| 7 | Pseudomonas aeruginosa
ATCC 27853 |
22 mm | 12mm | 11mm | 10mm* | —– | —– | 220.82 |
| 8 | Bacillus subtilis
ATCC 6633 |
—– | —– | —– | —– | —– | —– | |
| 9 | Staphylococcus aureus
ATCC 25923 |
—– | —– | —– | —– | —– | —– | |
| 10 | Enterococcus faecalis
ATCC 29212 |
17 mm* | —– | —– | —– | —– | —– | 171.41 |
| 11 | Aspergillus flavus
MTCC 277 |
—– | ||||||
| 12 | Candida albicans
MTCC 3017 |
—– | ||||||
| 13 | Candida tropicalis
MTCC 184 |
—– | ||||||
| 14 | Candida glabrata
MTCC 3019 |
45 mm | 32mm | 27mm | 20 mm | 10mm* | 451.41 | |
| 15 | Trychophyton rubrum MTCC 3272 | —– | ||||||
| 16 | Microsporum gypseum MTCC 4479 | —– | ||||||
| 17 | Epidermophyton floccosum
MTCC 613 |
—– | ||||||
mm- millimeter; mg- milligram; ml- milliliter and * indicates minimum inhibition concentration
 |
Figure 1: Antimicrobial activity of Boerhaavia diffusa (Punarnava).
|
According to Girish et, al. (2008), the methanol extracts of Boerhaavia diffusa had wider range of activity than the aqueous extracts, on the following bacteria viz., Bacillus cereus, Bacillus megaterium, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus, Streptococcus faecalis and Yersinia enterocolitica which indicates that the methanol extracts of all selected plants may contain the active components. In the present study aqueous extract (decoction) of Boerhaavia diffusa was found active against seven bacteria including Escherichia coli, Salmonella typhi, Salmonella typhimurium, Klebsiella pneumoniae, Enterobacter aerogenes, Pseudomonas aeruginosa and Enterococcus faecalis (Plate1 and 2). Thus the sensitivity of these microorganisms indicates that the decoction may also contain the active components and also supports the use of this plant as to cure many diseases like diarrhoea, infections of intestinal tract, throat and ear, fever, skin diseases, febrifuge, ophthalmic infections, jaundice, strangury, leucorrhoea, inflammation, cough and cold, eye and heart troubles and tertial fever (Bhattacharjee 1998 and Khare 2007). Boerhaavia diffusa is reported to posses many pharmacological, clinical and antimicrobial properties. It is one of the constituents of Liv 52 syrup for liver disorders (Himalaya Drug Company), Atishol tablets for amoebiasis and diarrhoea, Herboliv tablets and syrup for jaundice, liver cirrhosis and worm infestation and Raktojin tablets for worm infestation and anaemia (Vyas Pharmaceuticals), Punarnavarishta for kidney function and restorative (Dabur India Ltd.), Ural capsules and syrup for urinary tract infections and other urinary complaints, Vasuliv tablets and syrup for liver dysfunction, jaundice and viral hepatitis (Vasu Pharmaceuticals), Livo syrup and tablets for jaundice (Anjani Pharmaceuticals) etc. Recently the authors Awasthi et, al. (1979) observed potent antiviral efficacy of this plant against phytopathogenic viruses. The antiviral agent isolated from this plant was found to be a glycoprotein with a molecular weight of 16–20 kD. Administered by foliar spraying in the field, this antiviral agent could protect some economically important crops against natural infection by plant viruses.
 |
Plate 1
|
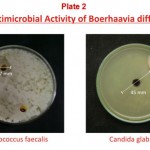 |
Plate 2
|
Acknowledgements
It gives us immense pleasure to express our special appreciation to Shri S. G. Koparkar Guruji for providing information on Ayurveda, Ethnomedicine and Naturopathy. The authors gratefully acknowledge Dr. Archana Nikam, a reputed Ayurvedic practitioner and Dr. V. G. Thakare, Principal, Shri Shivaji Science College, Amravati, for providing the laboratory and library facilities during the course of the research work.
References
- Awasthi LP and Verma HN. 1979. Boerhaavia diffusa– A Wild Herb With Potent Antimicrobial Properties. Canadian J of Botany. www.agri-history.org/pdf/Boerhaavia.pdf
- Bhattacharjee SK. 1998. Handbook of Medicinal Plants. Pointer Publishers, Jaipur. Pp 58-343.
- Girish HV and Satish S. 2008. Antibacterial Activity of Important Medicinal Plants on Human Pathogenic Bacteria – A Comparative Analysis. World Applied Sciences Journal. 5 (3): Pp 267-271.
- Khare CP. 2007. Indian Medicinal Plants: An Illustrated Dictionary Springer (India) Pvt. Ltd. Pp. 88-663.
- Mishra AS. 1989. Bhaishajyakalpana-Vigyan. Chaukhamba Surbharati Prakashan, Varanasi. Pp. 167-172.
- Saeed S and Tariq P. 2007. Antibacteial activities of Emblica officinalis and Coriandrum sativum against gram negative urinary pathogens. Pak. J. Pharm. Sci. Pp. 31-34.

This work is licensed under a Creative Commons Attribution 4.0 International License.





