Manuscript accepted on : November 20, 2009
Published online on: 28-12-2009
Abdellah Akhkha
Department of Biology, Taibah University, Al-Madinah Al-Munawwarah Kingdom of Saudi Arabia.
Corresponding Author E-mail: abdellah99@gmail.com
ABSTRACT: The objective of these experiments was to examine how water deficit stress affects the physiological processes in a desert plant species Calotropis procera. Photosynthesis, respiration and chlorophyll index were examined in order to determine how much changes in these processes could be involved in the reductions of dry matter production observed in our laboratory. It was found that water deficit did not affect the rate of photosynthesis at low water regimes (30%) compared to the 80% water regime. In contrast, moderate water regime (50%) increased the level of photosynthetic rate as a compensatory mechanism. Dark respiration was increased by the 30% water regimes and dramatically lowered by the 50% water regime. Chlorophyll content measured as chlorophyll index was increased in both low and moderate water regimes, explaining why the photosynthesis rate was not affected and suggesting that water deficit stress does not affect the photosynthetic machinery. Mechanisms involved in protecting the photosynthetic machinery and its efficiency from water deficit stress are discussed.
KEYWORDS: Water stress; photosynthesis; Chlorophyll index; Calotropis procera
Download this article as:| Copy the following to cite this article: Akhkha. A. The Effect of Water Stress on Photosynthesis, Respiration and Relative Chlorophyll Index of the Desert Plant Calotropis Procera. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Akhkha. A. The Effect of Water Stress on Photosynthesis, Respiration and Relative Chlorophyll Index of the Desert Plant Calotropis Procera. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8821. |
Introduction
Water plays a very important role in all physiological processes in plants including photosynthesis, respiration, translocation, partitioning of metabolites, stomatal behaviour, protein synthesis, cell division, cell elongation and cell wall synthesis. Water stress will lead to the perturbation of all or some of these physiological processes and consequently will lead to reductions in plant growth and yield (Ingram & Bartels, 1996).
Desert plant species are well known to withstand harsh environmental factors such as water deficit stress but keeping their metabolism active by the regulation of the osmotic and water relations in order to survive (Khan & Beena, 2002).
Desert plant species are well known to develop morphological adaptations that enable them to survive under harsh environmental conditions (Altesor & Ezcurra, 2003; Perea et al., 2005). However, in contrast to crop plants, our understanding of the physiological and biochemical responses of desert species to water deficit is much more limited (Van Heerden et al., 2007).
It is well known that water deficit stress reduces the rate of photosynthesis in many desert species. The mechanism(s) behind such reduction is not well understood. However, stomatal closure is considered as the major cause for photosynthesis decline when plants are under water deficit stress (Cornic, 2000; De Souza et al., 2005). Other mechanisms are also reported such as the inhibition of the activity of the enzyme Ribulose-1,5-bisphosphate Carboxylase/Oxygenase known as Ribusco (Parry et al., 2002).
The effects of different water regimes on photosynthesis, respiration and chlorophyll content were determined in a desert plant species Calotropis procera, which is well adapted to arid regions environment such as AlMadinah Almunawwarah. As far as we know, the present work is the first to study the effects of water deficit on the photosynthetic rates of C. procera.
Materials and Methods
Plant Material & Growth Conditions
Mature fruits of Calotropis procera were collected from plants grown in the region of AlMadinah AlMunawwarah, Kingdom of Saudi Arabia. The fruits were placed in paper envelops and left to dry under room temperature conditions for 2 weeks. Seeds were collected, washed and then soaked in distilled water for 24 hours prior to germination. Seeds were placed on distilled water saturated filter paper in Petri dishes and then incubated in the dark at 30ºC ± 2ºC. Seedlings of uniform size were planted in plastic pots filled with compost and sand mixture (3:1). The pots were placed in a growth cabinet at a temperature 28ºC ± 2ºC. Illumination was provided by fluorescent tubes lamps giving 150 µmol quanta m-2 s-1 at plant level during 16 hours photoperiod. The relative humidity within the cabinet was between 40-50 %.
Photosynthesis and Respiration Measurements
Rates of photosynthesis and respiration were measured in the forth leaf of 4 weeks old Calotropis plants using LI-COR 6400XT Infra Red Gas Analyzer (LI-COR Biosciences, 4647 Superior Street, Lincoln, NE 68504, USA). All measurements were made at constant temperature of 30ºC ± 2ºC. Illumination was provided by the LICOR light source, which allows the selection of a range of different light levels ranging from 0 to 1500 µmol quanta m-2 s-1. The CO2 concentration was kept constant at 350 µmole using the LI-COR 6400XT CO2 Mixer.
Chlorophyll Levels Determination
Chlorophyll levels in Calotropis plants were determined as Chlorophyll Index using a non-destructive method that uses an optical instrument called Chlorophyll Meter (Apogee Instruments Inc., 721W 1800N, Logan, UT 84321, USA).
Data analyses
Analysis of variance (ANOVA) was performed on all the data using Minitab version 11.0. The values presented are the means of three replicates ± S.E.
Results and Discussion
Photosynthesis rate was measured in the forth leaf of plants treated with one of the three water regimes (30%, 50% and 80%). The results are summarised plotted graphically in Figs 1, 2 & 3.
Effect of water stress on the photosynthesis rate
The results in Fig. 1 show that 30% of the field capacity water regime had no significant (p > 0.05) effect on the photosynthetic rate compared to the well watered plants at 80% water regime. This shows that the photosynthetic machinery in Calotropis plants was not affected by low water regime suggesting that this species is well adapted to water deficit stress and chloroplasts can function normally at very low levels of water regimes. This was the case in cotton plants (Massacci et al., 2007), but not the case in some other crop plants such as wheat (HongBo et al., 2005). How can some plants protect their photosynthetic machinery against water deficit stress is not fully understood; however, Massacci et al. (2007) suggested that the photosynthetic electron transport is promoted during the onset of water deficit stress in tolerant plants due to a higher efficiency of the open PSII reaction centers. In the present work we suggest that the extra energy is used either to keep the photosynthesis rate constant as in low water regimes (30%) or even to enhance photosynthesis rate as in the case of moderate water regimes (50%) (Fig. 2). The involvement of photorespiration in the observed findings is not investigated; however, some authors believe that water deficit stress increases the capacity of photorespiration in order to prevent an over-decline of the photosynthetic machinery (Massacci et al., 2007).
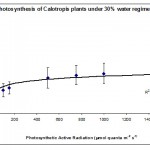 |
Figure 1
|
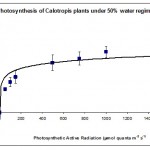 |
Figure 2
|
Fig. 2 shows that 50% water regime had the highest rate of photosynthesis compared to the other 2 water regimes suggesting a sort of compensatory photosynthetic mechanism that is induced when plants are under moderate water stress. Such mechanism is well known when plants are suffering from biotic stresses (Akhkha, 2009).
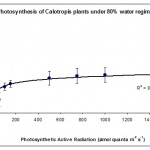 |
Figure 3
|
Chlorophyll Content
Chlorophyll content was measured as Chlorophyll Index using the Chlorophyll Meter (see Materials & Methods). Fig. 4 shows that extreme and mild water regimes 30% and 50% respectively had a highly significant (p < 0.01) effect on chlorophyll content measured as chlorophyll index. Both regimes increased the chlorophyll content in the leaves in order to cope with the water deficit stress and to compensate for any effect that could affect the function of the photosynthetic machinery. This phenomena act as a defence mechanism against abiotic stresses that can be detrimental to the plants. How this desert species preserve the photosynthetic machinery and tolerate water deficit stress is not known and more work has to be carried out to understand such mechanism(s) in order to use it for future crop plants to withstand shortage of water especially in arid regions such as the Kingdom of Saudi Arabia. In other studies, the level of chlorophyll and chlorophyll a + b ratio were not affected by water deficit (Munné-Bosch et al., 2006). In contrast, Guerfel et al. (2009) reported that water deficit decreased the level of chlorophyll (a + b) content in different Tunisian olive varieties (Olea europaea L.). The decrease in chlorophyll due to water deficit was attributed to the sensitivity of this pigment to such stress, which has been reported by several researchers (Younis et al., 2000; Moran et al., 1994). In the present study, Calotropis plants showed high insensitivity of the chlorophyll pigment to water stress suggesting certain mechanism(s) protecting the photosynthetic machinery from such stress. We conclude that Calotropis procera plants are highly tolerant to water deficit stress which explains the insensitivity of its growth in very harsh environments such as drought; this makes it a very successful species in arid regions such as Saudi Arabia.
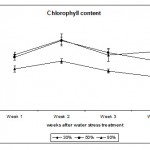 |
Figure 4
|
Dark Respiration
Dark respiration was also measured under absence of light (0 µmol quanta m-2s-1) and the results are shown in Fig. 5. The results show that low water regime 30% increased significantly (p < 0.05) the rate of dark respiration to about a third. However, at 50% water regime dark respiration was dramatically decreased (p < 0.001) to about 80% of of the control water regime. These results suggest that the mechanism, by which the photosynthetic rate in moderate water regime is creased, is through lowering the rate of dark respiration and reduce the level of energy utilized under water deficit stress. In contrast, other studies showed no change in the dark respiration due to water deficit (Ribas-Carbo et al., 2005). However, the same study observed that severe water regimes caused a significant shift of electrons from the cytochrome to the alternative pathway. This was not investigated in the present study and no conclusions can be made concerning the involvement of the alternative pathway.
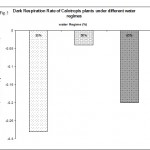 |
Figure 5
|
Conclusion
It was concluded in the present study that the rate of photosynthesis was not affected even at low water regime (30%) indicating the ability of Calotropis plants to withstand water limitations. Furthermore, Calotropis was found to increase its rate of photosynthesis at mild water regime (50%) indicating a sort of compensatory photosynthetic mechanism(s) that enable the plants to have high adaptability to harsh environment such as water deficit. This explains why this species grow very well in arid regions without growth limitation. How the photosynthetic machinery is protected against water stress is not known; however, more work was carried out in a different study, to investigate the efficiency and effectiveness of photosystem II and its integrity by using chlorophyll fluorescence technique (Akhkha, 2009).
References
- Akhkha, A. (2009). Chlorophyll Fluorescence of the Desert Plant Calotropis procera Grown under Water Deficit Stress. Bioscience, Biotechnology Research Asia, 6(2): in press.
- Akhkha, A. 2008. The effect of powdery mildew (Blumeria graminis f.sp. hordei) on compensatory photosynthesis and dark respiration in the uninfected fourth leaf of infected cultivated and wild barley lines. Bioscience, Biotechnology Research Asia, 5(2): 515-524.
- Altesor, A., Ezcurra, E. 2003. Functional morphology and evolution of stem succulence in cacti. J. Arid Environ. 53: 557–567.
- Cornic, G., 2000. Drought stress inhibits photosynthesis by decreasing stomatal aperture, not by affecting ATP synthesis. Trends Plant Sci., 5: 187-188.
- De Souza, C.R., Maroco, J.P., dos Santos, T.P., Rodrigues, M.L., Lopes, C., Pereira, J.S., Chaves, M.M., 2005. Control of stomatal aperture and carbon uptake by deficit irrigation in two grapevine cultivars. Agric. Ecosyst. Environ. 106: 261-274.
- Guerfel, M., Baccouri, O., Boujnah, D., Chaïbi, W., Zarrouk, M. 2009. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Scientia Horticulturae, 119: 257–263.
- HongBo, S., ZongSuo, L., MingAn, S., ShiMeng, S. and ZanMin, H. 2005. Investigation on dynamic changes of photosynthetic characteristics of 10 wheat (Triticum aestivum L.) genotypes during two vegetative-growth stages at water deficits. Colloids and Surfaces, 43: 221-227.
- Ingram, J., Bartels, D., 1996. The molecular basis of dehydration tolerance in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 47: 377-403.
- Khan, M. A. and Beena, N. 2002. Seasonal variation in water relations of desert shrubs from Karachi, Pakistan. Pak. J. Bot. 34(4): 329-340.
- Massacci, A., Nabiev, S.M., Pietrosanti, L., Kematov, S.K., Chernicova, T.N. and Leipner, J., 2008. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under filed conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiology and Biochemistry, 46(2): 189-195.
- Moran, J.F., Becan, M., Iturbe-Ormaetyl, I., Frechilla, S., Klucas, R.V., Aparicio-Tejo, P., 1994. Drought induces oxidative stresses in pea plants. Planta, 194: 346-352.
- Munne´-Bosch, S. and Cela, J. 2006. Effects of water deficit on photosystem II photochemistry and photoprotection during acclimation of lyreleaf sage (Salvia lyrata L.) plants to high light. Journal of Photochemistry and Photobiology, 85: 191–197.
- Parry, M.A.J., Andralojc, P.J., Khan, S., Lea, P.J., Keys, A.J., 2002. Rubisco activity: effects of drought stress. Ann. Bot. 89: 833-838.
- Perea, M.C., Ezcurra, E., Léon de la Luz, J. L., 2005. Functional morphology of a sarcocaulescent desert shrub in the bay of La Paz, Baja California Sur Mexico. J. Arid Environ. 62: 413–426.
- Ribas-Carbo, M., Taylor, N. L., Giles, L., Busquets, S., Finnegan, P. M., Day, D. A., Lambers, H., Medrano, H., Berry, J. A. and Jaume Flexas. 2005. Effects of Water Stress on Respiration in Soybean Leaves. Plant Physiology, 139: 466–473.
- Van Heerden, P.D.R., Swanepoel, J.W., Krüger, G.H.J. 2007. Modulation of photosynthesis by drought in two desert shrub species exhibiting C3-mode CO2 assimilation. Environmental and Experimental Botany, 61: 124–136.
- Younis, M.E., El-Shahaby, O.A., Abo-Hamed, S.A., Ibrahim, A.H., 2000. Effects of water stress on growth, pigments and 14CO2 assimilation in three sorghum cultivars. Agron. Crop Sci., 185(2): 73–82.

This work is licensed under a Creative Commons Attribution 4.0 International License.





