Manuscript accepted on : May 03, 2009
Published online on: 28-12-2009
Application of Ozone to Control Insect Pests and Moulds of Date Fruits
Saeed S. Al-Ahmadi, Reda A. Ibrahim and Salama A. Ouf
Biology Department, Faculty of Science, Taibah University, Medina Munawarah, P.O. 30002 Saudi Arabia.
ABSTRACT: In this study, the effectiveness of gaseous ozone and ozonated water on mycological flora and insect pests of ten date fruit cultivars were investigated. Steady reduction of fungal count was achieved at 8 ppm ozone gas applied for 4 hours in the case of most test plant materials reaching 2.2 – 6.6 as compared to 48.3-126.0 colonies/g, in the case of control. The longer-term exposure (6 hours) was lethal for all contaminating fungi. The elimination of insect pests requires higher ozone doses where the percent infestation at 30 ppm ozone applied for 6 hours ranged from 0.00 to 4.00% as compared with 7.85 and 15.68% in the case of control. Ozonized water applied for 2 minutes, at the same doses was more effective than gaseous ozone form and induced complete elimination of fungi and insect pests. The spore yield of Alternaria alternate, Aspergillus flavus, Paecilomyces divaricata and Penicillium citinum were reduced by 77.88, 81.56, 79.96 and 81.31%, respectively when exposed to 6 ppm for 4 hours. The total mycotoxins produced by Fusarium oxysporum, P. citrinum and A, flavus were significantly reduced on ozonation. The percent of unhatched eggs of Oryzaephilus surinamensis and Cadra furcatella, two of the insect pests manifesting fruit damage, reached 97.26 and 83.67%, respectively at 5 ppm ozone applied for one hour. Treatment of date fruits of different cultivars with ozone resulted in slight missing of some individual sugar fractions from some cultivars and varying reduction on percent relative concentration of sugar components.
KEYWORDS:
Ozone; insect (pests and mould); date fruits
Download this article as:| Copy the following to cite this article: Al-Ahmadi S. S, Ibrahim. R. A, Ouf. S. A. Application of Ozone to Control Insect Pests and Moulds of Date Fruits. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Al-Ahmadi S. S, Ibrahim. R. A, Ouf. S. A. Application of Ozone to Control Insect Pests and Moulds of Date Fruits. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8616 |
Introduction
Date fruits are the most important agricultural export commodities of Saudi Arabia. The conventional date production method leads considerable quality and quantity losses caused by microbial pathogens, mycotoxins and insect infestations (Al-Ahmadi et al. 2009). As even low level of insect or fungal infestations could spread very rapidly in improper storage conditions, suitable precautions should be taken to reduce spoilage risks. Therefore, the need for potent and safe antimicrobial and pesticidal agents has increased in recent years. Ozone is known to act as strong antimicrobial agent against bacteria, fungi, viruses and insect pests. It is a powerful oxidant that has numerous beneficial applications.
Ozone can be applied to foods as a gas or dissolved form in water. Main purposes of ozone application is the inactivation of bacterial growth (Sharma et al., 2002; Achen and Yousef, 2001; Kim and Yousef, 2000; Xu, 1999), prevention of fungal decay (Palou et al., 2002; Perez et al., 1999), destruction of pesticides and chemical residues (Hwang et al., 2001; Ong et al., 1996) controlling of storage pests (Mendez et al., 2003; Kells et al., 2001), degradation of aflatoxin from agricultural products (McKenzie et al., 1998). However, no universally applicable, effective and practical methods are currently available (Peltonen et al., 2000). Moreover, limited research has been performed on reduction of aflatoxins and insect pests control by ozone treatment in dried fruits.
The objective of this study was to evaluate the effectiveness of ozone gas and ozonized water to control insect infestation and to retard the mycotoxins production by some of the isolated fungi. The detection of the change in fruit’s active constituents as a result of ozone treatment will be also evaluated.
Materials and methods
Test samples
Fruits of ten cultivars of dates namely, Barni Mabroom, Baeiddy, Shalaby, Safawy, Sokai, Ajwah, Anbrah, Rabbiah and Rothana were used in this study. The samples were collected, in sterile plastic bags, from different localities in Madinah , Saudi Arabia, and stored in refrigerator until use.
Ozone production
Ozone was generated via a controlled flow of oxygen through a corona discharge in the ozone generator (Ozomaxe, Egypt, ozo- 3vtt). Ozone measurement was done by an ozone analyzer (Inusa, H1, ver 5.73) with a detection limit of 1-100 ppm.
Fungal isolation
Isolation of fungi was carried out from the naturally contaminated dates before and after ozone treatment. Czapek–Dox’s agar media (Dox, 1910) was used as isolation medium. The dilution plate method as described by Johnson et al., (1960) was adopted for counting of fungi. Pieces (1 cm X 1 cm) of untreated tissues or treated with 8 ppm ozone gas for 240 minutes or 8 ppm ozonized water for one and two minutes were dipped momentarily into a 0.5 % (m/v) calcium hypochlorite solution and four pieces (about 10 g) were mixed with 90 ml of sterile distilled water and shaked vigorously. Suitable dilutions were made for each plant sample. After solubilization and sterilization of the medium, streptomycin 30 mg/ml was added. Fifteen ml of this medium were cooled to just above the solidification and added to each Petri-dish. One ml from the prepared dilution of each plant sample was transferred aseptically into each of six petri-dishes containing isolation medium. The dishes were rotated by hand in a broad swirling motion so that the diluted samples were dispersed in agar. After incubation at 28oC for 7 to 15 days, the resulting colonies were estimated per gram dry material. The developing fungal colonies were identified up to the species level by microscopic examination. This was made through the help of the references of Barnett (1960), Barron (1968), Ellis (1971,1976), Kendrick (1971), Moubasher (1993), Raper and Fenell (1965), Samson (1979), (Pitt, 1979), Klich and Pitt (1982) and Robert et al. (1996).
Insect rearing
The insects infesting date fruits were recovered under the insectary conditions (25±2ºC, 75±5% R.H. and 16h of illumination per day) from naturally infested dates, either untreated or treated with 30 ppm ozone gas for 360 and 480 minutes or 30 ppm ozonized water for one and two minutes. The fruits were placed in plastic pots (15 cm diameter and 20 cm deep). The pots were then covered with muslin or cheese-cloth fastened by a rubber-band to prevent the escape of insects and to ensure the proper ventilation. The emerged insect species obtained from the culture were counted and the percent infestation rate calculated as the number of infested date fruits manifesting typical insect damage, 45 days after incubation per 100 fruits / replicate was estimated.
Sporulation
Plates of Czapek Dox agar were inoculated, each with 6 ppm ozone- treated inoculum of each test fungus. The inoculum was exposed for 0, 60, 120, 180, and 240 min. The plates were then incubated for 7 days at 28ºC. A 1 cm2 section was cut from the margin of the colony and transferred to a vial containing 10 ml sterile distilled water. The suspension was continuously shaken for 5 minutes and the concentration of spores per ml was counted in a hemocytometer. Three plates were used for each treatment. Non ozone treated inoculums was used as a control.
Mycotoxins production
The method suggested by Christian (1990) was used. HPLC analysis was used to detect and determine the Aflatoxins, B1, B2, G1 and G2 from the samples which homogenized with 10 ml of solution (methanol: 0.0001M Sodium nitrate, 50:50, v:v) . The extract was passed through micro-filter 0.45 µm. The analysis of aflatoxins was performed on HPLC model (HP1050). HPLC equipped with UV detector. The separation and determination were performed on C18 column (150 x 4.6 mm). The mobile phase yielded results of (methanol : 0.0001M Sodium nitrate, 50:50, v : v). The wave length in the UV detector was 365 nm and the total run time for the separation was approximately 15 min at a flow rate of one ml/min.
In this experiment three mycotoxigenic fungi were selected, namely, Aspergillus flavus, Fusarium oxysporum, and Penicillium citrinum. These fungi were exposed to ozone gas at concentration 6ppm for 0-240 min.
Aflatoxins of Aspergillus flavus
About 10 gm of biomass of tested culture, which grow on PDA, with 1 gm salt (NaCl) and place in blender jar. 100 ml methanol: water (80:20, v/v) was added and mixture blended for 1min. The extract put into fluted filter paper and the filtrate was collected in clean vessel. Ten ml of filtered extract was diluted with 40 purified water and mix well. The dilute extract was filtered through glass microfibre. Analysis of aflatoxins was performed on a model ‘HP1050′ HPLC equipped with UV detector. Separations and determinations were performed on RP18 (ODS) column (length150mm). The mobile phase was methanol: acetic acid: water (20:20:60 v/v/v) and wave length was 365 nm while flow rate was 1 ml/min according to Joshua (1993).
Patulin and penicillinic acid toxins of Penicillium citinum
About 10 gm of biomass of tested culture which grow on PDA with 10 ml of solution acetonitrile: water (6/94, v/v) place in blender jar. The mixture blended for 1 min. The extract put into micro-filter 45μm and the filtrate was collected in clean vessel. Extraction of penicillinic acid was taken the same trend of patulin extraction but without extraction solution of acetonitrile: water (60:40 v/v). Analysis of patulin was performed on a model ‘HP1050′ HPLC equipped with UV detector. Separations and determinations were performed on RP18 (ODS) column (length 250 mm).
Toxins of Fusarium oxysporum
Fumonisin analysis
Fumonisin toxins were determined according to the method described by Mazzani et al., (2001). In this method fifty gm of ground sample (biomass) with 5 gm salt and 100 ml methanol: water (80:20) were blended at a high speed for one min., and then filtered through fluted filter papers. Ten ml of the filtrate was diluted with 40 ml of wash buffer and filtered again through 1.0 μm micro-fiber filter. Ten ml of the diluted extract was passed through fumontest column (Vicam Company) and then the column was washed by 10 ml of the same dilute solution. The fumonisin was eluted by pass one ml of HPLC grade methanol through the column and the elutes were re-collected again. One ml of developer A (Vicam product No. G5005) and developer B (vicam product No. G5004) was added to the elute and place in calibrated fluorometer (Series-4 / Vicam).
Zearalenone analysis
The zearalenone concentration was determined as mentioned in the fumonisin toxin but the dilution was made with 49 ml distilled water which passed through Zeara test column (Vicam Company) and then measured in calibrated fluorometer model (Series-4/Vicam) according to the method of Martins et al., (2003).
Egg hatchability
The preliminary results revealed that Oryzaephilus surinamensis and Cadra furcatella were the most dominant insect recovered from date fruits. For mass production of eggs, both insect pests were reared on date fruits under the insectary conditions described before. The effect of 1-5 ppm ozone gas delivered for one hour on hatchability of one and three day-old eggs was examined. Untreated eggs were used for comparison. Five replicates, each of 50 eggs, were used for each treatment. One week after treatment, both treated egg ages were daily investigated and dead one and/or malformed were recorded and segregated. Hatching percentage was determined.
Stability of sugar constituents of the test fruits
Two grams of each sample were socked separately in 75% ethanol (100 ml). After 24 hr., the sample was ground and filtered. The residue was washed with a few ml of 75% ethanol and the volume was made up to100 ml. Several sugars were examined using a HPLC system (HP1050) with a UV detector at 190 nm. The separation was accomplished with APS column (5 µm. 4 x 250 mm). The mobile phase consists of (acetonitril / water; 76/24 v/v.). The flow rate was 1.0 ml/min. while, the injection volume was 10 μl according to the method of (Christian, 1990).
Statistical analysis was performed for all experiments by using ANOVA multiple mean comparisons.
Results
Fungal and insect infestaion
Table 1 indicates variable effectiveness of ozone against the population of fungi inhabiting the test plant materials. Steady reduction of fungi was achieved at 8 ppm ozone gas applied for 4 hours in the case of most test plant materials reaching 2.2 – 6.6 as compared to 48.3-126.0 colonies/g, in the case of control. Complete elimination of fungi after 6 hours exposure, was achieved in the case of date fruit cultivars of Safawy, Sokai, and Labban. The longer-term exposure (6 hours) at 8 ppm ozone gas was lethal for all contaminated fungi.
Ozonized water was more effective when applied at 8 ppm for one minute and induced sterilization for most test fruits including Barni, Safawy, Sokai, Ajwah, Anbrah, and Lebban. Two minute- exposure induced 100% elimination of fungi from all test date fruits.
The percent infestation rate recovered from the different cultivars of dates manifesting typical insect damage, after their treatment with 30 ppm ozone gas or ozonized water indicates complete reduction in infestation for 4 cultivars namely Shalaby, Anbrah Rothan and Labban (Table 2). The percent infestation of the other cultivars ranged from 1.05 to 4.00% as compared with 12.09 and 15.68% in the case of control, respectively. Ozonized water applied for one minute induced 100% dis-infestation for all test date fruits except Barni and Rabbiah (1.38 and 0.50 as compared to 12.09 and 7.85% for control).
Complete eradication of insect pests from date fruits for all cultivars was achieved using ozone gas applied at 30 ppm for 8 hours or 30 ppm ozonized water for 2 minutes.
Sporulation
Due to the failure of most test fungi to grow at 8 ppm, so the effect of ozone on sporulation was studied only at 6 ppm.
The data in Fig 1 reveal that, the extension of exposure time of the inoculum to ozone gas from 60 to 240 minutes induced a progressive decrease in production of spores. The inoculum of A. niger and F. oxysporum failed to grow when treated with 6 ppm ozone for 240 minutes. The spore yield of A. alternate, A. flavus, P. divaricata and P. citinum were reduced by 77.88, 81.56, 79.96 and 81.31%, respectively when exposed for 240 minutes.
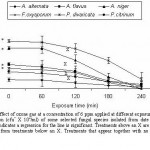 |
Figure 1
|
Mycotoxins production
In this experiment three species, selected from the isolated fungi, were tested to determine the effect of their exposure to ozone on mycotoxins production. These species are well-known as mycotoxins producers and include; Aspergillus flavus, Fusarium oxysporum and Penicillium citrinum. The inoculum of each fungus was treated with ozone at 6 ppm for 60, 120, 180 and 240 minutes before inoculating to the growth medium.
Under control condition, Aspergillus flavus produces 4 aflatoxins (AFB1, AFB2, AFG1and AFG2), F. oxysporum produces fumonisin and zearalenone, and P. citrinum produces potuline and penicillc acid (Table 3). A. flavus was the most mycotoxin producer where the total estimated aflatoxins reached 155.02µg/g dry mass. F. oxysporum produced 29.20 µg/g dry mass while P. citrinum was the least mycotoxins producer (27.51µg/g dry mass).
In case of F. oxysporum and P. citrinum, the production of mycotoxins gradually decreased with the extension of the exposure time to reach a minimum value for the mycelium originating from inoculum exposed for 180 minutes. At this condition, the total mycotoxins accounted 6.28 and 4.71 µg/g dry mass, in the cases of F. oxysporum and P. citrinum as compared to 29.20 and 27.51 µg/g dry mass under control condition, respectively. A. flavus was less susceptible to ozone although there was a steady decrease in mycotoxin production with the gradual increase in ozone concentration reaching 85.56, 38.56, 20.34 and 14.18 µg/g when exposed to 60, 120, 180 and 240 minutes, respectively as compared with 155.02 for control.
Hatchability
Results in Fig 2 showed that hatching of eggs of both O. surinamensis and C. furcatella decreases with increasing ozone dose applied for one hour. At 5 ppm ozone concentration, the unhatched eggs was 81.00 and 97.26% for the first species and was 83.67 and 87.98% for second species, in the case of one and three-day-old eggs, respectively. Generally, the three–day old eggs of O. surinamensis were more sensitive to ozone than that of one-day-old eggs, while the eggs of C. furcatella exhibited a reverse condition. It was noted that the non-viable eggs were darkened, shrinked, flaccid and sunken on side after ozone treatment.
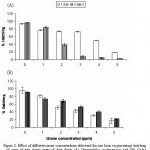 |
Figure 2
|
Stability of sugar constituents
Date fruits of different cultivars contain varying levels of sugar constituents. Fourteen sugar components were detected in Barni and Baeiddy, thirteen in shalaby and rabbiah, eleven in Ajwahand, Rothan and Labban, nine in Sokai, eight in Safawy and Anbrah (Table 4). Sucrose, fructose and glucose constituted the bulk of sugar fractions. Maximum percent of relative concentration of sucrose (57.12%) was detected in Anbrah, of fructose (32.03%) in Sokai and of glucose (13.03%) in Rabbiah (Table 5).
The application of ozone at 30 ppm for 8 hours induced disappearance of some sugar fractions and varying reduction on percent relative concentration of sugar components. Gluconic acid, sorbose and sorbitol were missed on application of ozone from Sokai, Anbarah and Rabbiah, respectively. The quantitative reduction in sugar fractions ranged from 17.36, 17.37 and 17.38 for Barni, to 0.87, 0.87 and 0.90 for Shalaby in the case of sucrose, fructose and glucose, respectively.
Discussion
The research indicates variable effectiveness of ozone against the population of fungi and insect pests inhabiting the test plant materials. The sensitivity of contaminating fungi to ozone may be affected by several factors including the method of application, strain of the microorganism, growth level, nature and water content of date ‘ s tissue and quantitative amount of sugars. Complete elimination of fruits infesting fungi was achieved after 6 hours exposure of 8 ppm ozone gas or 2 minutes exposure of the same dose in water. Kim et al. (1993) found that ozone treatment (6 mg/liter/s for 60 minutes) decreases the counts of Salmonella spp, Staphylococcus aureus, Bacillus cereus, Penicillium spp., or Aspergillus spp contaminating black peppercorns. Ozone is thought to kill microorganisms by oxidation of cellular components such as sulphydryl groups and amino acids of enzymes, peptides, proteins and polyunsaturated fatty acids, and oxidation of the cell membrane (Victorin, 1992; Xu, 1999; Young and Setlow, 2004; Das et al., 2006). Degradation of unsaturated lipids of the cell membrane by ozone was resulted in cell disruption and subsequent leakage of cellular contents. Due to potent destruction and damage of nucleic acids, cellular death could be occurred (Escriche et al., 2001; Das et al., 2006).
The efficacy of ozone in eliminating fungal and insect pests depends on its applied form, contact period, infesting species and fruit cultivar. Ozonized water is more effective than gaseous ozone even when applied for one minute and two-minutes contact with the test tissues was enough to induce sterilization. Ozone has also been shown to have potential to kill insect pests in commodities (Erdman, 1980, Mason et al., 1997 L.J. Mason, C.P. Woloshuk and D.E. Maier, Efficacy of ozone to control insects, moulds and mycotoxins. In: E.J. Donahaye, S. Navarro and A. Varnava, Editors, Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products, Nicosia, Cyprus, Printco Ltd., Nicosia (1997), pp. 665–670.Mason et al., 1997 and Kells et al., 2001). High mortality was achieved for adults of the maize weevil, Sitophilus zeamais Motschulsky, and the confused flour beetle Tribolium confusum du Val, and the larval stage of the Indian meal moth, Plodia interpunctella (Hübner) exposed to low ozone concentrations ranging from 5 to 45 ppm (Erdman, 1980 and Kells et al., 2001). Erdman (1980) also observed mortality of larvae of T. confusum and the red flour beetle, Tribolium castaneum (Herbst) when exposed to a 45 ppm ozone environment. Leesch (2003) tested ozone as a toxicant to stored-product insects in the hope of killing insects at low dosages in short periods of time. In his study, even high concentrations of 200–500 ppm (v/v) required many hours to kill the insects exposed. Işikber and Öztekin (2009) indicated a remarkable difference in susceptibility between the life stages of Ephestia kuehniella and Tribolium confusum for ozone treatment resulting in complete mortality of adults, pupae and larvae, while only 62.5% of the eggs were killed.
The spore production of all test fungi was reduced on exposure to 6 ppm ozone and the reduction was more pronounced on extension of exposure time. The maximum reduction in spore production was achieved in the case of A. flavus and P. citinum exposed for 240 minutes where it accounted 0.52 and 0.37 X 106 cfu/ml, as compared to 2.82 and 1.98 X 106 cfu/ml in the case of control, respectively. The inoculums of A. niger and F. oxysporum failed to form spores at 6 ppm ozone exposure for 240 minutes. The efficacy of ozone to suppress fungal sporulation is well documentated in reports of Palou et al. (2003), Mason et al (1997), Krause and Weidensaul (1978), and Harding (1968). Heagle and Strickland (1972) observed distortion and plasmolysis of conidia when exposed to 0.2 ppm ozone and suggested that ozone might entered directly into the conidia or conidiophore. Rahman Khan and Wajid Khan (1998) working with Sphaerotheca fuliginea observed smaller conidia (in length and width) after post-inoculation exposure at 0.2 or 0.1 ppm ozone.
Since some of the isolated fungi in this research are known to be mycotoxin producers, so it is important to determine the effect of their exposure to ozone on mycotoxins production. For this purpose Aspergillus flavus, Fusarium oxysporum and Penicillium citrinum, recovered from the investigated cultivars (Al-Ahmadi et al., 2009), were selected for this purpose. A. flavus was more susceptible to 6 ppm ozone where the reduction of aflatoxins reached 90.85%after 240 minutes, as compared with 82.88% and 78.49% in the case of P. citinum and F. oxysporum, respectively exposed to the same dose for 180 minutes. A. flavus could not able to produce AFG2 and AFG1 when exposed to 6 ppm for 120 and 180 minutes respectively.
Several research studies have been undertaken to evaluate the effects of ozone gas in reducing aflatoxin levels in contaminated agricultural products. Inan et al. (2007) stated that the high oxidising power of ozone achieved detoxification of aflatoxin. They subjected the contaminated samples to ozonation at various ozone concentrations (16, 33, 66 mg/l) and exposure times (7.5, 15, 30, 60 min) and recorded 80% and 93% reductions of content of aflatoxin B1 in flaked and chopped red peppers after exposures to 33 mg/l ozone and 66 mg/l ozone for 60 min, respectively. Zorlugenç (2008) indicated that degradation of aflatoxin B1 increased with increasing of ozonation time and the gaseous ozone was more effective than ozonated water for reduction of aflatoxin B1, whereas ozonated water was affected for decreasing microbial counts.
Our results indicate that the toxic effect of eggs and hatching depends on the species and egg age. The toxic effect of ozone at high doses may be due to modification of the eggshell protein polymer by the oxidant, rendering it more resistant to hatching enzyme (Gromol et al. , 2003).
Treatment of date fruits of different cultivars with ozone resulted in slight missing of some individual sugar fractions from some cultivars and varying reduction on percent relative concentration of sugar components. An et al. (2007) indicated changes in lignifying, antioxidant enzyme activities and cell wall composition of fresh-cut green asparagus pretreated with 1mg/l aquuueous ozone. The enzyme activities in fresh-cut asparagus including phenylalanine ammonia lyase, superoxide dismutase, ascorbate peroxidase, glutathione reductase were inhibited by aqueous ozone treatment. The investigators were also monitored changes in lignin, cellulose and hemicelluloses content.
In a similar study, Zhao and Cranston (1995) stated that ozone treatment of ground black pepper resulted in slight oxidation of volatile oil constituents but ozone had no significant effect on the volatile oils of whole peppercorns.
Acknowledgment
This work was kindly supported by the Taibah University Scientific Research Program.
References
- Achen, M., and Yousef, A.E. ( 2001). Efficacy of ozone against Escherichia coli 157:H7 on apples. Journal of Food Science, 66: 1380–1384.
- Al-Ahmadi, S. S., Ibrahim, R. A. and Ouf, S. A. (2009). Possible control of fungal and insect infestation of date fruits using ozone. Biosciences, Biotechnology Research Asia, 6. In press.
- An, J., Zhang and M., Lu, Q. (2007). Changes in some quality indexes in fresh-cut green asparagus pretreated with aqueous ozone and subsequent modified atmosphere packaging. J. Food Engineering, 78: 340-3444
- Barnett, H. L. (1960). Illustrated genera of imperfect fungi. Burgess publishing company, Minneapolis, pp. 225.
- Barron, G. L. (1968). The genera of Hyphomycetes from soil. Williams and Wilkins, Baltimore.
- Christian, G. (1990). HPLC Tips and Tricks. Great Britain at the Iden Press, Oxford .pp 608.
- Das, E., Candan and G., Bayındırlı, A. (2006). Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella enteritidis on cherry tomatoes. Food Microbiology, 23: 430–438.
- Dox, A. W. (1910). The intracellular enzymes of Penicillium and Aspergillus species, special refrence to those of P. camenberiti U.S. Department of Agriculture and Animal Bulletin, 120, 170.
- Ellis, M. B. (1971). More Dematiaceous Hyphomycetes. Commonwealth, Mycol. Inst,, Kew.
- Ellis, M. B. (1976). Dematiaceous Hyphomycetes. Commonwealth, Mycol. Inst,, Kew.
- Erdman, H.E. (1980). Ozone toxicity during ontogeny of two species of flour beetles,
- Tribolium confusum and T. castaneum. Environmental Entomology, 9: 16–17.
- Escriche, I., Serra, J.A., Gomez, M. and Galotto, M.J. (2001). Effect of ozone treatment and storage temperature on physicochemical properties of mushrooms (Agaris bisporus). Food Science and Technology International, 7: 251–258.
- Grotmol, S., Dahl-Paulsen and Totland, G.K. (2003). Hatchability of eggs from Atlantic cod, turbot and Atlantic halibut after disinfection with ozonated seawater. Aquaculture, 221:245-254.
- Harding, P.R. (1968). Effect of ozone on Penicillium mold decay and sporulation, Plant Disease Rep., 52: 245-247.
- Hwang, E.S., Cash, J.N. and Zabik, M.J. (2001). Postharvest treatments for the reduction of mancozeb in fresh apples. Journal of Agricultural Food Chemistry, 49: 3127–3132.
- Inan, F., Pala, M. and Doymaz, I (2007). Use of ozone in detoxification of aflatoxin B1 in red pepper. Journal of Stored Products Research 43: 425-429.
- Isikber, A. A., and Oztekin, S. (2009). Comparison of susceptibility of two stored-product insects, Ephestia kuehniella Zeller and Tribolium confusum du Val to gaseous ozone. Journal of Stored Products Research. In press.
- Johnson, R. L., Curl. Bond, J. and Priboury, H. (1960). Method for studying soil microflora-Plant Disease Relationships. Burgess Publishing Co., Minneapolis, 178.
- Joshua, H. (1993) Determination of aflatoxins by reversed-phase high performance liquid chromatography with post column in line photochemical derivitization and fluorescence detection. Journal of Chromatography A, 654: 247-254.
- Heagle, A. S. and Strickland, A. (1972). Reaction of Erysiphe graminis f. sp. horedi to low level ozone. Phytopathology 62: 1142-1148.
- Kells, S.A., Mason, L.J., Maier, D.E. and Woloshuk, C. (2001). Efficacy and fumigation characteristics of ozone in stored maize. Journal of Stored Products Research 37, 371–382.
- Kendrick, B. (1971). Taxonomy of fungi Imperfect. Toronto university, Canada.
- Kim, M. J., Oh, Y. A., Kim, M. H., Kim, M. K. and Kim, S. D. (1993). Fermentation of Chinese cabbage kimchi inoculated with Lactobacillus acidiphilus and containing ozone- treated ingredients. Journal of Korean Society and Food Nutritiob, 22: 165-174.
- Kim, J.G. and Yousef, A.E. (2000). Inactivation kinetics of foodborne spoilage and pathogenic bacteria by ozone. Journal of Food Science, 65: 521–528.
- Klich, M.A. and Pitt. J.I. (1992) A laboratory guide to common Aspergillus species and their teleomorphs. Published by Commonwealth Scientific and Industrial Research Organization, Division of Food Processing.
- Krause, C. R. and Weidensaul, T. C. (1978). Effects of ozone on the sporulation, germination and pathogenicity of Botrytis cinerea. Phytopathology, 68: 195-197.
- Leesch, J.G. (2003). The mortality of stored-product insects following exposure to gaseous ozone at high concentrations. In: Credland, P.F., Armitage, D.M., Bell, C.H., Cogan, P.M., Highley, E. (Eds.), Advances in Stored Product Protection, Proceedings of the 8th International Working Conference on Stored-Product Protection, 22–26 July 2002, York, UK. CAB International, Oxon, UK, pp. 827–831.
- Martins H. M. and Bernardo F. (2003). Fungal flora and mycotoxins detection in commercial pet food. Revista Portuguesa DE Ciencias Veterinarias, 98: 179-183.
- Mazzani C., Borges O., Luzon O., Barrientos V. and Quijada P. (2001). Occurrence of Fusarium moniliforme and fumonisins in kernels of maize hybrids in Venezuela, Brazilian Journal of Microbiology, 32: 345-349.
- Mckenzie, K. S., Kubena, L. F., Denvir, A. J., Rogers,T. D., Hitchens, G. D., Bailey, R. H., Harvey, R. B., Buckley, S. A. and Philips, T. D. (1998). Aflatoxicosis in turkey poults is prevented by treatment of naturally contaminated corn with ozone generated by electrolysis. Poultry science, 77: 1094-1102.
- Mason, L.J., Woloshuk, C.P. and Maier, D.E. (1997). Efficacy of ozone to control insects, moulds and mycotoxins. In: Donahaye, E.J., Navarro, S., Varnava, A. (Eds.), Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products. Nicosia, Cyprus Printer Ltd., Nicosia, pp. 665– 670.
- Moubasher, A. H. (1993). Soil fungi in Qatar and other Arab countries. Scientific and Applied Research Center, university of Qatar. pp. 566.
- Mendez, F., Maier, D.E., Mason, L.J., Woloshuk, C.P. (2003). Penetration of ozone into columns of stored grains and effects on chemical composition and processing performance. Journal of Stored Products Research, 39: 33–44.
- Ong, K.C., Cash, J.N., Zabik, M.J., Siddiq, M. and Jones, A.L. (1996). Chlorine and ozone washes for pesticide removal from apples and processed apple sauce. Food Chemistry 55, 153–160.
- Palou, L., Crisosto, C.H., Smilanick, J.L., Adaskaveg, J.E. and Zoffoli, J.P. (2002). Effect of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biology and Technology, 24: 39–48.
- Palou, L., Smilanick, J. L., Crisosto, C.H., Mansour, M., Plaza, P. (2003). Ozone gas penetration and control of sporulation of Penicillium digitatum and Penicillium italicum within commercial packages of oranges during cold storage. Crop Protection, 22: 1131-1134.
- Peltonen, D., El-Nezami, H.S., Salminen, S.J. and Ahokas, J.T. (2000). Binding of aflatoxin B1 by probiotic bacteria. Journal of the Science of Food and Agriculture 80, 1942–1945.
- Perez, A., Sanz, C., Rios, J.J., Olias, R. and Olias, J.M. (1999). Effects of ozone treatment on postharvest strawberry quality. Journal of Agricultural Food Chemistry, 42: 1652–1656.
- Pitt, J.I. (1979). The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press London, New York, Sydney.
- Raper, K. and Fennell, D. I. ( 1965). The Genus Aspergillus. Willians and Wilkins Co. Baltimore.
- Rahman Khan, M,, and Wajid Khan, M. (1998). Interactive effects of ozone and powdery mildew (Sphaeotheca fuliginea) on bottle gourd (Lagenaria siceraria). Agriculture, Ecosystems & environment 70: 109-118.
- Robert, A.S., Hoekstra, Frisvad, J.C. and Filtenborg, O. (1996) Introduction to food-borne fungi. Printed by Ponsen and Looyen, Wageningen, The Netherlands.
- Samson, R. A. (1979). A compilation of the Aspergillus described since 1965. C. B. S. Stud. Mycol. 18.
- Sharma, R.R., Demirci, A., Beuchat, L.R. and Fett, W.F. (2002). In activation of Escherichia coli O157:H7 on inoculated alfalfa seeds with ozonated water and heat treatment. Journal of Food Protection, 65: 447–451.
- Victorin, K. (1992). Review of genotoxicity of ozone. Mutation Research 227, 221–238.
- Xu, L. (1999). Use of ozone to improve the safety of fresh fruits and vegetables. Food Technology, 53: 58–63.
- Young, S. and Setlow, P. (2004). Mechanism of Bacillus subtilis spore resistance to and killing by aqueous ozone. Journal of Applied Microbiology, 96: 1133–1142.
- Zhao, J. and Carnston, P. M. (1994). Microbial decontamination of black pepper by ozone and the effect of the treatment on volatile oil constituents of the spice. Journal of Science and food Agriculture, 8: 11-15.
- Zorlugenc, B., Zorlugenc, F.K., Oztekin, S. and Evliya, B. (2008). The influence of gaseous ozone and ozonated water on microbial flora and degradation of aflatoxin B1 in dried fig. Food and Chemical Toxicology, 46:3593-3597.

This work is licensed under a Creative Commons Attribution 4.0 International License.





