Manuscript accepted on : August 30, 2009
Published online on: --
Antioxidant and Anti-Tyrosinase Activity of Lime Extracts
R. Sariri* and H. Ghafoori
Department of Biology, Faculty of Science, University of Guilan, Rasht Iran.
Corresponding Author e-mail: : sariri@guilan.ac.ir
ABSTRACT: Formation of melanin in fruits and vegetables damaged by mechanical injury during harvesting, postharvest storage or processing is the main reason for browning. Oxidation of phenolic compounds to the corresponding quinines, facilated by tyrosinase, is responsible for this enzymatic browning. Although the use of synthetic compounds such as organic acids, amines, thiols and most recently, alcohols as tyrosinase inhibitors have been studied and reported, natural inhibitors have received less attention in this regard. The present study reports the free radical scavenging and anti-tyrosinase activity of whole lime and dried peel extracts. It was found that lime extracts contained ascorbic acid ranging from 60.52 to 85.64 mg/g DW, gallic acid from 10.30 to 28.25 mg/g DW, and ellagic acid from 8.25 to 11.50 mg/g DW depending on the cultivars. Whole lime extracts contained higher levels of all three plant polyphenols and also exhibited the highest radical scavenging activities when compared with dried peel extracts.
KEYWORDS: Tyrosinase; inhibition; enzyme kinetics; whole lime extract
Download this article as:| Copy the following to cite this article: Sariri R, Ghafoori H. Department of Biology, Faculty of Science, University of Guilan, Rasht (Iran). Biosci Biotechnol Res Asia 2008;6(2). |
| Copy the following to cite this URL: Sariri R, Ghafoori H. Department of Biology, Faculty of Science, University of Guilan, Rasht (Iran). Biosci Biotechnol Res Asia 2008;6(2). Available from: https://www.biotech-asia.org/?p=20114 |
Introduction
Enzymatic browning of plants damaged by mechanical injury during harvesting, postharvest storage or processing is the main cause of their quality loss1-2. Tissue browning in fruits and vegetables is predominantly catalyzed by tyrosinase, the a copper-containing enzyme also called as catecholase (EC.1.14.18.1)3-5. In general, polyphenol oxidases (PPO) (EC 1.14.18.1) are oxido-reductase enzymes capable of catalyzing hydroxylation of monophenols and the subsequent oxidation of o-diphenols to o-quinones6-8. The enzymatic products, o-quinones, are susceptible to oxidation, leading to polymerization and the formation of brown, red or black pigments5-6. The most important endogenous phenolic substrates for PPO in apple and potato sources are catechol, L-3, 4-dihydroxyphenylalanine (L-DOPA), caffeic acid, 4-methylchatechol, 4-hydroxyphenylpyruvic acid, p-coumaric acid and m– and p-cresol9. Polyphenol oxidase mediated browning in raw fruits and vegetable has been known as the major cause of quality deterioration in fruits and vegetables and their derived food products10. Other oxidizing enzymes, such as peroxidase (EC 1.11.1.7) can also catalyze enzymatic browning. The relative contribution of these two enzymes is still unknown and may differ with plant source11-14. Design of a chemical reagent and strategy to control enzymatic browning is a challenge to the food industry15-19. Tyrosinase inhibitors are, therefore, important in food industry as their activity is of importance in preventing synthesis of melanin in the browning of plants and fruits.
A number of synthetic thiol compounds have been reported to act as tyrosinase inhibitors20. These –SH containing compounds can also act as potent inhibitors on peroxidases21-22. On the other hand, inhibition of mushroom tyrosinase by other compounds has also been studied. It has been stated that the kinetics of inhibition depends highly on the type of substrate23. A number of organic compounds including aromatic carboxylic acids24, sulphites25, amino acids26 and aromatic amines27 have been identified as effective inhibitors of PPO. However, the use of these compounds has been restricted due to their potential hazards. In addition, the use of browning inhibitors in food processing is restricted by considerations relevant to wholesomeness, and effect on taste, flavour, texture, and cost. Browning inhibitors may be classified in accordance with their primary mode of action. In general, a number of compounds have been studied for prevention of enzymatic browning. These include (1) reducing agents; (2) acidulants; (3) chelating agents; (4) complexing agents; (5) enzyme inhibitors and (6) enzyme treatments28. Lime is typically round, green in color, 3–6 cm in diameter, generally containing sour pulp. Limes are often used to accent the flavors of foods and beverages. Two species of lime are mostly grown in north of Iran, Persian lime (Citrus latifolia) and West Indian (C. aurantifolia). In this study, free radical scavenging and inhibitory activity on mashroom tyrosiase by dried peel and whole lime extracts cultivated in north of Iran was investigated. 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical was used as active scavenger of free radicals. The progress of enzymatic reaction was followed using 3-methyl-2-benzothiazolinone hydrazone (MBTH) as the color reagent. The product of tyrosinase activity on dopamine hydrochloride reacted with the amino group in MBTH to produce a deep pink colored complex with a maximum absorption at 503 nm.
Materials and Methods
Materials
Mushroom tyrosinase, dopamine hydrochloride and DMF were purchased from Sigma Chemical Company. Sodium mono-phosphat, sodium di-phosphates, propylene glycol, DMSO, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and MBTH were obtained from Merck representative in Iran. Nylon membrane (13 mm) was obtained from Orange Scientific, Belgium. Lime fruits were freshly harvested from the field (Faculty of Agriculture, University of Guilan, Rasht, Iran).
Assesment of enzyme purity by electrophoresis
Polyacrylamide gel electrophoresis (SDS-PAGE) was used as a tool to examine the purity of the enzyme. The purity of enzyme is of prime importance for the kinetics studies. The electerophoresis conditions have been explained previously29. The gel was stained with colloidal brilliant blue and de-stained in acetic acid-methanol mixture.
Preparation of the enzyme and substrate solution
a) Enzyme solution: Pure mushroom tyrosinase (1mg/ml) was used without further purification and diluted to 1/160 of its original concentration.
b) Substrate solution: Dopamine hydrochloride (44 mM) was freshly prepared in phosphate buffer (pH 6.8) containing 2% (v:v)DMF and 5 mM MBTH. To prevent its color change by the action of direct light, this solution was stored in dark until use.
Lime fruit samples
Lime fruits were freshly harvested from the field in November 2008 (Department of Horticulture, Faculty of Agriculture, University of Guilan, Rasht, Iran) and transported to laboratory of Biochemistry (Faculty of Sciences, University of Guilan). A selection of the fruits was washed and separated into 2 groups, one was peeled and the peels dried in the oven at 50 °C for 48 h and the other group was used as whole fruit. After drying, the peels were ground into fine powder. In the second group, the whole fresh fruits were homogenized in a food processor.
Preparation of lime extracts
500 g of dried lime peel powders were extracted with 2 L of hot water (50°C) for one hour. The extract was then filtered and collected. The residue was re-extracted twice with 2 L of hot water each time. The three water extracts were combined, concentrated and lyophilized using a freeze-dryer. The whole lime fruits (peel, pulp and seed) were homogenized in a food processor and extracted with hot water (50°C, extraction ratio 1:5, w/w) for 3h, filtered, and then lyophilized; these fruits were only extracted once with water. The percentage yield of extracts was between 12.5% and 18% depending on cultivars and plant tissues samples. For HPLC analysis, plant extracts, about 20mg, were dissolved in 4.0ml of hot water (50°C) (3 replicates per sample) and mixed thoroughly. They were left at room temperature until to cool down and filtered through a 0.45 μm Nylon membrane (13 mm)30. To study the antioxidant activity, samples were dissolved in DMSO (100%) at a final concentration of 5 mg/ml prior to chemical assay. Finally, 3-6 mg/ml samples were prepared in phosphate buffer (pH 6.8) for tyrosinase inhibition studies.
Scavenging of diphenyl-picrylhydrazyl (DPPH) radicals
2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical was used as active scavenger of free radicals in the extracts. The reduced DPPH formazan form was determined using a spectrophotometer. A modification of the assay method described by van Amsterdam et al31 was used. In a typical experiment, 5ml of each sample or 100% DMSO (as a negative control) or 10 mM ascorbic acid (as a positive control as well as a blank for background subtraction) were allowed to react with 195 μl of 100 μM DPPH aqueous solution in a 96-well micro-plate. The plate was then incubated at 37°C for 30 min after which the absorbance was measured at 515 nm using a UV–VIS micro-plate reader. Scavenging capacity of the sample was compared to that of DMSO (0% radical scavenging) and ascorbic acid as positive control (100% radical scavenging). The results expressed as the concentration of the extracts or pure compounds which scavenged free radicals by 50% (SC50).
Inhibition of tyrosinase activity
The diphenolase activity of mushroom tyrosinase was measured spectrophotometrically using kojic acid as a standard tyrosinase inhibitor. The enzymatic reaction was initiated by addition of a known amount of the enzyme to a solution of substrate containing dimethyl formamide (DMF and MBTH). DMF was added to the reaction mixture in order to keep the resulting colored complex in soluble state during the course of investigations. The progress of the reaction was followed by measuring the intensity of the resulting pink color at 505 nm. A typical reaction mixture with a total volume of 1.0 ml contained 100 ml enzyme solution (a), 500 ml substrate solution (b) and 400 ml phosphate buffer (pH 6.8). To investigate the effect of natural inhibitors on the activity of enzyme, tyrosinase activity was measured by replacing the phosphate buffer with 400 ml of lime extracts (3-6 mg/ml). The 50% inhibition (IC50) of tyrosinase activity was calculated as the concentrations of each sample that inhibited 50% of tyrosinase activity. The data were expressed as a percentage of inhibition of tyrosinase activity.
Results
HPLC analysis showed the presence three polyphenolic compounds, ascorbic, gallic and ellegic acid in the lime extracts. The chromatogram obtained by reverse phase HPLC is presented in Figure 1. The concentration of phenolic compounds was measured in the presence of standards and the results are presented in Table I. The values of poly phenols are reported in terms of milligrams of the compound per grams of dried weight (DW) of sample, as compared to known concentrations of each pure compound as standard. The whole lime extract contained considerably higher levels of these compounds than dried peels in both species. It can be seen that lime extracts contained ascorbic acid ranging from 54.32 to 85.64 mg/g DW, gallic acid from 9.45 to 28.25 mg/g DW, and ellagic acid from 6.55 to 11.50 mg/g DW. Each sample was run triplicate on HPLC and the results repoted as the mean value.
Table 1: The contents of polyphenols in different lime extracts (quantitative HPLC results).
| Extract | Ascorbic acid
(mg/g DW) |
Gallic acid
(mg/g DW) |
Ellagic acid
(mg/g DW) |
Total polyphenols (mg/g DW) |
| Whole C. latifolia
C. latifolia peel Whole C. aurantifolia C. aurantifolia peel |
85.64 ± 2.2
59.65 ± 0.3 78.50 ± 1.2 54.32 ± 0.08 |
28.25 ± 1.4
10.88 ± 0.06 25.35 ± 0.6 9.45 ± 0.2 |
11.50 ± 0.8
6.98 ± 0.4 10.45 ± 1.3 6.55 ± 0.3 |
140.55 ± 1.2
78.45 ± 0.8 124.97 ± 1.4 72.25 ± 0.6 |
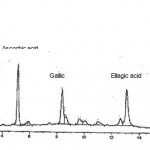 |
Figure 1: HPLC chromatogram of the polyphenolic acids present in lime extracts showing their relative contributions.
|
Free radical scavenging ability of lime extracts (DPPH assay)
It is known that polyphenols are good scavengers of free radicals. DPPH∙ is a stable free radical that is able to accept one hydrogen radical to become a stable diamagnetic molecule, yellow coloured diphenylpicrylhydrazine32. The DPPH∙ scavenging capacity of these extracts may be mostly related to their phenolic hydroxyl groups. HPLC analysis confirmed that whole lime extracts contained high levels of gallic, ellagic and ascorbic acids, while the dried peel extracts had lower contents of these poly phenolic acids. In the present study whole lime and dried peel extracts were evaluated for their abilities to neutralize the stable free radicals such as DPPH radicals. The SC50 values (the concentration that scavenges 50% of the DPPH radical) for the whole and peel extracts were between 8.7 and 75.3 μg/ml depending on the plant species and tissue used for preparation of extract. In addition, standard pure ascorbic, gallic and ellagic acid showed very high activity towards DPPH radicals with SC50 2.0, 2.4 and 2.9 μg/ml, respectively (Table II). It is known that the total antioxidant activity of the each extract is the sum of the individual activities of each phenolic compound present, and also that these compounds might have synergistic effects. Dorman et al.33 demonstrated that the OH∙ scavenging activities does not depend on the total phenolic content. They found that free radical scavenging activity of rosemary and thyme extracts were similar. However, total phenolic content of rosemary was 2-fold higher than thyme.
Table 2: Total free radical scavenging activities (DPPH assay) of lime extracts and some pure reference standards.
| Extracts or pure standards | DPPH assay SC50 (μg/ml) |
| Whole C. latifolia
C. latifolia peel Whole C. aurantifolia C. aurantifolia peel Ascorbic acid Gallic acid Ellagic acid |
9.7 ± 0.2
18.7 ± 3.2 12.6 ± 0.5 19.3 ± 0.6 2.0 ± 0.1 2.4 ± 0.1 3.9 ± 0.2 |
Inhibition of tyrosinase activity
Both whole lime and lime peel extracts showed anti-tyrosinase activity depending on their concentrations, i.e. plant source and lime species (Figure 2). The IC50 values for whole peel lime extracts were 2.1 and 3.6 mg/ml, respectively. However, the inhibitory activity of these extracts was weaker than a reference inhibitor such as kojic acid (IC50 = 8.9 μg/ml). We have previously found that ascorbic acid is a potent tyrosinase inhibitor34. The total inhibitory activity exhibited by lime extracts is, therefore, mainly related to high content of ascorbic acid.
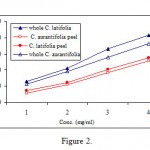 |
Figure 2: Anti-tyrosinase activity of whole lime and lime peel extracts. The numbers on Y axes refer to 0.5, 1.0, 1.5 and 2.5 mg/ml concentrations respectively. |
Discussion
The reactive oxygen species (ROS) include superoxide anion radicals (O2∙-), hydrogen peroxide (H2O2), and hydroxyl radicals (OH∙) and accumulation of these ROS can result in oxidative stress that has been related to human diseases such as cardiovascular diseases, cancers, aging, diabetes, and atherosclerosis35. Polyphenolic compounds including ascorbic, gallic and ellagic acid have possess strong radical scavenging activity36. It can be expected, therefore, in addition to their use in food industry to prevent enzymatic browning of food products, plant extracts can be used in traditional medicine. In other words, plant extracts containing high levels of free ascorbic, gallic and ellagic acid may be able to scavenge excessive free radicals such as superoxide anion radical (O2∙-) and peroxyl radical (ROO∙) in the body and protect human cells or tissues against oxidative stress. The results showed that whole lime extracts contained higher levels of three major polyphenolic compounds, ascorbic, gallic and ellagic acid, than dried peel (Table I). In this study, multiple extraction and mechanical process (stirring 1 h each time, 3 times) to increase the efficiency of the extraction method. Considering that phenolic compounds are partially non-polar, it is expected that adding some percent of non-polar solvent may add the extraction yield. Both gallic acid and ellagic acid were less potent scavengers than ascorbic acid in DPPH assay. These two compounds have also been reported to be responsible for the scavenging activity of Terminalia chebula extract in the DPPH assay37. The results of this study showed that lime peel extract containing lower levels of ascorbic, gallic and ellagic acid exhibited less radical scavenging activity than whole lime extract that contained higher levels of these compounds. However, in the DPPH assay, the SC50 values of whole lime and dried peel extracts (C. latifolia and C. aurantifolia) were 8.7 and 55.7 μg/ml repectively. These results indicate that contents of phenolic acids present in the concentrated lime extracts are much lower than the SC50 of individual pure compound (2.3 and 2.2 μg/ml, respectively). Therefore, individual phenolic acids may not be the only contributors to the high antioxidant effects of lime extracts. Considering the results obtained from the present study, it is expected that other polyphenolic/flavonoid glycosides or ellagitannins in plants, which can be easily extracted in hot water, may also contribute to the potent antioxidant activity of these extracts. It was found that, whole lime extract scavenged DPPH, superoxide (O2∙-), and peroxyl (ROO∙) radicals. The O2∙- and ROO radicals are among the most important free radicals which can be generated as harmful by-products implicating in lipid peroxidation and some diseases38. The ROO∙ radical is generated in normal metabolic reactions by all aerobic organisms and is also the source of the highly biologically reactive, hydroxy radical (OH∙). Therefore, free radicals scavengers present in natural plant extract can be effective in preventing a living organism against oxidative stress. In addition, whole lime extract had significant antityrosinase activity, although, its activity was lower than kojic acid. These results suggest that whole lime extract may be developed further as a natural source of free radical scavenging phytochemical. It could also find a wide application as a natural skin-whitening agent in cosmetic products.
Conclusions
The results obtained in the present study indicate that the substances present in whole lime extract are natural inhibitors for the reaction of tyrosinase on dopamine hydrochloride, its most common substrate. On the other hand, free radical scavenging properties of lime extracts may find use in preventing the oxidative stress. We are currently investigating the kinetics of other tyrosinase inhibitors present in many fruit extracts in the presence of other types of substrates and the results will be published soon.
References
- Sakuraka J., Takahashi S. and Hosoya J., J Biol Chem 261, 9657 (1968).
- Cilento G., and Adam W. Photochem. Photobiol. 48, 361 (1988).
- Mayer A.M. Phytochemistry 26, 11 (1995).
- Whitaker J.R., in Food enzymes: structure and mechanism (D.W.S. Wong eds), Chapman and Hall, New York, 271 (1995).
- Martynez M.V. and Whitaker J.R. Trends Food Sci. and Technol., 6(6), 195 (1995).
- Aspuru E.O. and Lourdes Zaton A.M., Spectrochimica Acta Part A 55, 2343 (1999).
- Prota G. Medical Research Reviews, 8, 525 (1988).
- Robbin D.A. and Lontie R. Copper proteins and copper enzymes Vol. II, Boca Raton, CRC Press, FL. 207 (1984).
- McEvily A.J., Iyengar R. and Otwell W.S. Food Technol., 45, 80 (1991).
- Michael Sullivan L. and Ronald Hatfield D. Crop. Sci., 46, 662 (2006).
- Lin Z., Chen L. and Zhang W. Process Biochem. 5, 443 (1996).
- Agostini E., Medina M.J., Siliva R., Forchetti M.D. and Tigier H. J. Agric. Food Chem., 45, 596 (1997).
- Everse J., Everse K.E. and Grisham M.B. Peroxidases in Chemistry and Biology, CRC Press, Boca Raton, FL. 1 (1991).
- Gaspar T., Penel C. and Greppin H. Plant Peroxidases 1980-1990, Progress and Prospects in Biochemistry and Physiology, (1992).
- Ballantyne A., Stark R. and Selman J.D. Int. J. Food Sci. Technol., 23, 267 (1988).
- Hicks K.B., Sapers G.M. and Seib P.A. U S Patent, 4, 975, 293, (1990).
- Tong C.B.S., Hicks K.B., Osman S.F., Hotchkiss A.T. and Haine R.M. J. Agri. Food Chem. 43, 592 (1995).
- La Mar G.N, Hernandez G. and Ropp J.S., Biochemistry 31, 9158 (1992).
- Zaton A.M.L. and Ochao de Aspuru E., FEBS Lett. 734, 192 (1995).
- Sariri R., Mahmoodian J. and Khaje Kh. Asian Journal of Chemistry, 18 (1) 8 (2006).
- Sariri R., Sajedi R.H., Jafarian V. and Khaje Kh. Journal of Molecular Liquids 123, 20 (2006).
- Sariri R., Sajedi R.H. and Jafarian V. Journal of Molecular Liquids 128, 175 (2006).
- Zollner H. Handbook of Enzyme Inhibitors Part A, Second revised and enlarged ed., VCH Verlagagesellschaft GmbH, Germany, 367 (1993).
- Chen Q., Song K.K., Qui L., Liu X.D., Huang H. and Guo H.Y., Food Chem. 91,269 (2004).
- Sayavedra-Soto, L.A., and Montgomery, M.W.J., Food Sci., 51,1531 (1986).
- Kahn V. and Andrawis A. Phytochemistry 24, 905 (1985).
- Gasowaska B., Kafarski P.and Wojtasek H. Biochimica et. Biophysica Acta 1673, 170 (2004).
- McEvily A.J, Iyengar R. and Otwell W.S., Crit. Rev. Food Sci. Nutr., 32, 253 (1992).
- Electrophoretic Theory, Hoffer Scientific Instruments, Electrophoresis Instruments. Techniques and Exercises (1990-1992) Catalogue.
- Rangkadilok N., Worasuttayangkurn L., Bennett R.N. and Satayavivad J., J. Agri. Food Chem., 53, 1387 (2005) .
- van Amsterdam F.T., Roveri A., Maiorino M., Ratti E. and Ursini F., Free Radical Biology and Medicine, 12, 183 (1992).
- Soares J R, Dins T C P, Cunha A P & Ameida L M (1997) Free Radical Research 26, 469–478.
- Dorman H.J.D., Peltoketo A., Hiltunen R. and Tikkanen M.J., Food Chem., 83, 255 (2003).
- Haghbeen K., Babaei M., Sariri R. et al., J Food Biochem (2008), In press, Accepted 21 May.
- Willcox J.K., Ash S.L. and Catignani G.L., 44, 275 (2004).
- Yilmaz Y. and Toledo R.T., J. Agri. Food Chem., 52, 255 (2004).
- Naik G.H., Priyadarsini K.I., Naik D.B., Gangabhagirathi R. and Mohan H., Phytomedicine, 11, 530 (2004).
- Gyamfi M.A. and Aniya Y. Biochemical Pharmacology, 63,1725 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.





