Manuscript accepted on : April 04, 2008
Published online on: 15-03-2016
Experimental Study of Biodiesel as Fuel for Wick Stove
N. Kapilan1, N. Vinay1 and Varun Jawahar Devur2
1Department of Mechanical Engineering, Nagarjuna College of Engineering and Technology Bangalore - 562 110 India.
2Department of Biotechnology, Nagarjuna College of Engineering and Technology Bangalore - 562 110 India.
Corresponding Author E-mail: kapil_krecmech@yahoo.com
ABSTRACT: There is an increasing interest in every country, to search for suitable alternative fuels that are environment friendly. This led to the choice of Mahua Oil (MO) as one of the main alternative fuels in India. In the present work, an attempt has been made to use biodiesel as a substitute for kerosene in wick stove. In this investigation, Mahua Oil Methyl Ester (MOME) was prepared by transesterfication using potassium hydroxide as catalyst and important fuel properties were determined based on ASTM procedure. From the analysis, it was observed that the properties of MEMO are close to kerosene. To evaluate the MOME as a substitute for kerosene, it was used as a fuel in wick stove. From the experimental results, it was observed that the MOME result in performance close to kerosene and lower soot emission.
KEYWORDS: Alternative fuel; Biodiesel; Wick Stove; Performance
Download this article as:| Copy the following to cite this article: Kapilan N, Vinay N, Devur V. J. Experimental Study of Biodiesel as Fuel for Wick Stove. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Kapilan N, Vinay N, Devur V. J. Experimental Study of Biodiesel as Fuel for Wick Stove. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7276 |
Introduction
In India, wood stoves and kerosene stoves are the main energy (heating) source for cooking in rural areas. The increase in wood consumption for cooking purposes results in deforestation, which leads to severe ecological, economical and sociological problems. Cooking stoves using fossil fuels like petroleum based gases or kerosene as energy source can economically and ecologically hardly be recommended in developing countries. Moreover, most tropical and subtropical countries are depending on fossil fuel imports. This often burdens the foreign currency reserves to a great extent. Because of the required infrastructure for distribution fossil fuels are expensive especially in remote areas. In addition, fossil fuels are becoming more depleted and prices are keep on rising. Utilization of biodiesel derived from non edible vegetable oils as cooking fuel presents a promising alternative and secures a sustainable and local available cooking energy supply.
Jamieson1 listed over 350 oil-bearing crops while Duke et al2 identified 70 species of oil seeds with considerable potential. Biodiesel obtained from vegetable oils has been considered a promising option for petroleum based fuels. Barnwal et al3 reviewed the work done on biodiesel production and utilization, resources available, processes developed, performance in existing engines, environmental considerations, the economic aspect, and advantages in and barriers to the use of biodiesel. Marchetti et al4 reviewed the alternative technological methods that could be used to produce biodiesel. They carried out different studies using different oils as raw material, different alcohol as well as different catalysts, homogeneous ones such as sodium hydroxide, potassium hydroxide, sulfuric acid and supercritical fluids, and heterogeneous ones such as lipases. They listed the advantages and disadvantages of technologies.
Subramanian et al5 discussed the policy and planning issues for utilization of ethanol and biodiesel in automotive diesel engines in Indian context in view of environmental benefits, energy self-sufficiency and boosting of the rural economy as well as measures related to implementation and barriers. Subhrabaran Das et al6 estimated the district-wise biomass residues generation and the potential for power generation from surplus biomass, in the state of west Bengal, India. The estimated total biomass residue generation in the West Bengal is 37.35mta and the agricultural residues constitute major share (79%) of the biomass residues generation. The total power generation potential from basic surplus biomass and net surplus biomass are about 2107 and 1197MW, respectively. Gupta et al7 made an attempt for financial analysis of cooking energy options in India using data from a field study and real costs and prices. They highlighted the need to alter energy policies to promote quality fuels and efficient devices in an accessible way to low income households.
Brinda et al8 analyzed the expenditure share of ‘clean’ and ‘dirty’ fuels in total cooking fuel consumption for the rural and urban households across 16 major states in India. Their study discussed the policies that could facilitate switch towards ‘clean’ fuels and argues that enabling policies should pay attention among other things to the gender issues and trade-offs that exist between say, local and global pollution, deforestation and resource depletion, and disease and subsidy burden. Pohekara et al9 reported that the energy requirements for cooking account for 36% of total primary energy consumption in India. They suggested that policy interventions required for better dissemination of renewable energy based devices. Natarajan et al10 made an attempt, to use waste vegetable oil as a fuel for a cooking stove. They made suitable modifications in the kerosene stove for use with vegetable oil as fuel. They reported that the efficiency of the stove using vegetable oil as fuel is observed to be as high as 48.9% as compared to 34.9% with that of a conventional stove when a flat copper bottom vessel is used.
Mahua name for a medium to larger tree, Madhuca longifolia of family Sapotaceae with wider and round canopy. The tree may attain a height of up to 20 meters. Mahua is a slow growing species, attains a mean height of 0.9 to 1.2 m at the end of the fourth year. The variety latifolia is common throughout the Indian sub-continent, including Bangladesh. It is a tree of deciduous nature, of the dry tropical and sub-tropical climate. As a plantation tree, Mahua is an important plant having vital socio-economic value. This species can be planted on roadside, canal banks etc on commercial scale and in social forestry programmes, particularly in tribal areas. The drying and decortification yield 70% kernel on the weight of seed. The kernel of seed contains about 50 % oil. The oil yields in an expeller is nearly 34 % – 37%. The fresh oil from properly stored seed is yellow in color11.
Shashikant et al12 developed a two step pretreatment technique to produce biodiesel from mahua oil having high free fatty acids. Sukumar Puhan et al13 used mahua oil ethyl ester as fuel in four stroke naturally aspirated direct injection diesel engine and studied the performance and emission of the diesel engine.
Utilization of the biodiesel cooking stove can produce numerous ecological, economical, and sociological benefits in developing countries. The biodiesel is a sustainable energy source ensuring sufficient cooking energy for adequate meal preparation. Introduction of the biodiesel cooking stove can be readily acceptable to people in tropical and sub-tropical countries since its operation is similar to the well-known kerosene stoves. In India, wick stove is used for heating and cooking purpose. Hence in the present work, an attempt was made to use biodiesel as substitute for kerosene in wick stove without any modification in its design.
Materials and methods
The cost of the MO is one fourth of the cost of the kerosene. Since MEMO is not produced commercially, its cost is higher than the kerosene. The MOME was prepared by transesterfication method. Figure 1 shows the transesterification reaction.
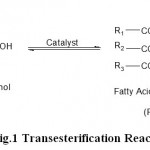 |
Figure 1: Transesterification Reaction.
|
In the present work, transesterification reaction was performed in a round bottom vessel of 500 ml in volume. Figure 2 (a) shows the experimental setup used for the transesterification. First, the vessel was filled with 210 ml of mahua oil. Then, measured amount of the methanolic potassium hydroxide, which was prepared by dissolving 2 gms of potassium hydroxide in 85 ml of methanol, was added to the reactor. For refluxing purpose, a vertical water cooled condenser was placed on the top portion of the vessel and the reactor was immersed in a constant-temperature water bath. The temperature of the water bath was maintained at 70 °C and agitation was provided with a magnetic stirrer during the reaction. This reaction was carried out for two hours. After the transesterification, the condenser was removed and the products were heated, to remove excess methanol. After heating, the products were shifted to 500 ml separator funnel, for phase separation. The top layer containing esters (biodiesel) were washed with warm water to wash out impurities like soap and other residues. Figure 2 (b) shows the MOME, MO and Glycerin. The properties of MO, MOME and kerosene were determined as per ASTM procedures. Table 1 shows the properties of MO, MOME and kerosene.
 |
Figure 2: (a) Experimental Setup (b) MEMO Mahua Oil Glycerin.
|
Table 1: Properties of MO, MOME and kerosene.
| Property
Flash point (°C) Fire point (°C) Calorific Value ( MJ/kg) Kinematic Viscosity at 40° C (cst) Density at 40°C (kg/m3) |
MO212 223 35.61 37.63 891 |
MOME
129 141 36.91 5.10 863 |
Kerosene
58 67 36.12 2.81 820 |
Experimental Setup
For the present work, a commercially available capillary-fed wick stove was used. Figure 3 shown the experimental setup used for the wick stove test. A mercury thermometer was used to evaluate the water temperature during experiment. An electronic weighing balance of accuracy 1 gm was used during the weight measurement of water and stove. A smoke meter was used for the measurement of smoke. The fuel was filled nearly three-fourth of the capacity in the fuel tank and an aluminum vessel was selected as per the fuel consumption test. A stirrer was used for uniform temperature rise of water.
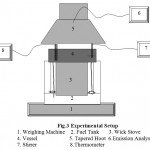 |
Figure 3: Experimental Setup.
|
Experimental Procedure
A match stick was used to light the wicks and after the warm up the weight of the wick stove was measured using a digital weighing machine. A known weight of vessel containing water was placed on the stove and the water was stirred for uniform heating. The time required for the temperature rise of water and emission was recorded. The weight of the stove after the experiment was recorded to determine the fuel consumption. Then the wick stove and wicks were cleaned with biodiesel and biodiesel was used as fuel. After warm up, the above procedure was followed for the biodiesel. The efficiency of the wick stove was found out with kerosene as fuel. Then the stove and wicks were cleaned with biodiesel. The biodiesel was filled nearly three-fourth of the capacity in the fuel tank and an aluminum vessel was selected as per the fuel consumption test.
Results and Discussion
The biodiesel fuelled wick stove was giving a stabilized flame similar to the kerosene. Figure 4 shows the flame produced by the biodiesel operated wick stove.
 |
Figure 4: Biodiesel operated Wick Stove.
|
The performance and emission of the wick stove was presented and discussed below. To study the behavior of the flames of the wick stove, flame produced by a single wick was studied. Figure 2 compares the flames of kerosene and MOME. From the figure, we can observe that there is a slight variation in the flame height of MOME and kerosene. The MOME results in slightly lower flame height. This may be due to the slightly higher viscosity of the MOME. The kerosene operation results in higher soot emission. This may be due to the higher volatility and incomplete combustion of the kerosene.
The thermal efficiency of the wick stove with kerosene and biodiesel are 51.82 % and 52.39% respectively. From these we observe that the biodiesel operation results in higher efficiency as compared to the kerosene. This may be due to the presence of oxygen in the molecular structure of the biodiesel which results in better combustion of the fuel. Since the kerosene operation produces more soot emission, it results in higher fuel consumption and lower efficiency. The fuel consumption rate of kerosene and biodiesel are 150.66 gm/hr and 145.8 gm/hr respectively.
The smoke opacity of the wick stove with kerosene and biodiesel are 22.6% and 16.3% respectively. The MOMO results in lower smoke emission than the kerosene. This may be due to the better combustion of the biodiesel which results in lower soot emission.
Conclusion
From the experimental results, the following conclusions are drawn. The biodiesel can be used as a substitute for kerosene in wick stove without any modification in its design. Only a small amount of ethanol is needed for start-up. The flame height of the biodiesel is comparable to kerosene. The biodiesel operation results in lower soot emission due to the presence of oxygen in its structure. The average thermal efficiency of the biodiesel is higher than the kerosene. Hence fuel economy can be achieved by the use of the biodiesel.
Acknowledgments
The financial help provided by Karnataka Council for Science and Technology, Government of Karnataka, India, is greatly acknowledged.
References
- Jamieson G S, Vegetable Fats and Oils, Reinhold Publishing Corporation, New York, 134-141, 1943.
- Duke J A and Bagby M O, Comparison of Oilseed Yields : A preliminary review in Vegetable Oil Fuels, ASAE Publication, ASAE, St.Joseph, M.I., 1982.
- Barnwal B K and Sharma M P, “Prospects of biodiesel production from vegetable oils in India”, Renewable and Sustainable Energy Reviews, 9 : 363–378 (2005).
- J.M. Marchetti, V.U. Miguel, A.F. Errazu, “Possible methods for biodiesel production”, Renewable and Sustainable Energy Reviews, 11 : 1300–1311 (2007)
- K.A. Subramanian, S.K. Singal, Mukesh Saxena, Sudhir Singhal, “ Utilization of liquid biofuels in automotive diesel engines: An Indian perspective”, Biomass and Bioenergy 29 : 65–72 (2005)
- Subhrabaran Das, Tushar Jash, “District-level biomass resource assessment: A case study of an Indian State West Bengal”, Biomass and Bioenergy (2008), doi:10.1016/j.biombioe.2008.05.001
- Gupta and N. H. Ravindranath, “Financial Analysis of Cooking Energy Options for India”, Energy Conservation Management 38(18) : 1869-1876 (1997).
- Brinda Viswanathan and K.S. Kavi Kumar, “Cooking fuel use patterns in India: 1983–2000”, Energy Policy 33 : 1021–1036 (2005)
- S.D. Pohekara, Dinesh Kumara, M. Ramachandrana, “Dissemination of cooking energy alternatives in India—a review”, Renewable and Sustainable Energy Reviews 9 : 379–393(2005)
- Natarajan, N.S. Karthikeyana, Avinash Agarwaala, K. Sathiyanarayanan, “Use of vegetable oil as fuel to improve the efficiency of cooking stove”, Renew Energy (2008), doi:10.1016/ j.renene.2008.01.022
- Bringi N V, “Non traditional Oil Seed and Oils of India”, Oxford and IBH Publishing Company Pvt Ltd, New Delhi, India, 57-58, 1987.
- Shashikant Vilas Ghadge and Hifjur Raheman, “Biodiesel production from mahua oil having high free fatty acids”, Biomass and Bioenergy, 28(6) : 601-605 (2005)
- Sukumar Puhan, N. Vedaraman, G. Sankaranarayanan and Boppana V. Bharat Ram, “Performance and emission study of Mahua oil ethyl ester in a 4-stroke natural aspirated direct injection diesel engine”, Renewable Energy, 30 (8): 269-1278 (2005)
- IS 2980:1999, Non-pressure stoves- specification, , edition 4.1, 2003-10.

This work is licensed under a Creative Commons Attribution 4.0 International License.





