Manuscript accepted on :
Published online on: 05-01-2016
Plagiarism Check: Yes
Non-Conventional Utilization Of Maize Husk For The Removal Of Iron From Industrial Wastewater
Mrunal M. Jogi1 and Ansari Ilyas A.2
1Department of Microbiology, P.S.G.V.P. Mandals ASC College, Shahada - 425 409 (India) . 2Universal Starch Chem Allied Ltd., Rawal Industrial Estate, Dondaicha - 425 408 (India).
ABSTRACT: The authors have utilized the maize husk, a byproduct of starch industries, without any treatment to study its feasibility as an inexpensive and effective adsorbent for the removal of iron from wastewater. Iron was adsorbed on the husk and the effect of various parameters such as particle size, pH, contact time and temperature was studied to ascertain the effective conditions for favourable removal of iron.
KEYWORDS: Maize Husk; Iron; adsorbent
Download this article as:| Copy the following to cite this article: Jogi M. M, Ilyas A. A.Non-Conventional Utilization Of Maize Husk For The Removal Of Iron From Industrial Wastewater. Biosci Biotechnol Res Asia 2003;1(1) |
| Copy the following to cite this URL: Jogi M. M, Ilyas A. A.Non-Conventional Utilization Of Maize Husk For The Removal Of Iron From Industrial Wastewater. Biosci Biotechnol Res Asia 2003;1(1). Available from: https://www.biotech-asia.org/?p=3380 |
Introduction
Heavy metal ions are toxic and carcinogenic in natures that have arrested the attention of researchers for ultimate removal to make the industrial effluent free from this hazard1-2. These metal ions can effectively be removed from sewage or industrial effluent by employing various methods like ion exchange, solvent extraction, reverse osmosis, co-precipitation, electrochemical reduction and adsorption3. Activated carbon and other adsorbents are widely used for adsorption study for removal of toxic metals4-5. However, these techniques are costly and their performance may hamper due to presence of other heavy ions. Therefore a quest for cheap treatment alternative has compelled the researchers especially of underdeveloped countries to adopt the most biodegradable agricultural source for removal of these metals6. There is a plethora of references available in the literature where the researchers have judiciously utilized ground nut husk, tea leaves7, tree bark, cotton capsule shells, rice straw, pea nut skin8 etc. for the removal of heavy metals from the effluent.
Iron picking effluents and mine water contain excessive amounts of iron9. Iron usually precipitates as hydroxide and forms heavy sludges in receiving streams as well as in treatment plants. The authors have made an attempt in this study to remove the iron from wastewater by employing maize husk, a byproduct of starch manufacturing industry.
Experimental
All the chemicals were of AnalaR grade. Double distilled water was used to prepare the solutions. Iron stock solution was prepared from ferrous ammonium sulfate crystals. The initial iron concentration in test solution was kept 50 mg/L throughout the investigation.
Maize husk was dried to almost zero moisture in oven, grounded and sieved through 20, 30 and 40 mesh size. The dried husk was stored in a desiccator during the study.
The batch adsorption study was conducted using iron solution and adsorbent with a fixed dose of 15 g/L at desired pH and temperature with predetermined contact time in the Erlenmeyer flasks. The flasks were shaken on mechanical shaker. The solutions were centrifuged and analysed for iron concentration.
The batch technique was adopted for kinetic study. The iron solution was shaken with a measured amount of adsorbent using water bath at constant temperature. At a predetermined time intervals, the solution was centrifuged and the supernatant was analysed for iron content. The equilibrium was reached in 75 min. The spectrophotometer method was used to determine the concentration of iron using 1, 10-phenanthroline. The analyses was carried out as per Standard Methods10. UV-Spectrophotometer of Shimadzu make (model UV-1601) was used in this study at 510 nm wavelengths. A pH meter of Elico make (model LI was used for pH measurements.
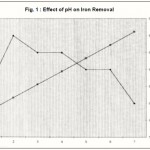 |
Figure 1 : Effect of pH on Iron Removal |
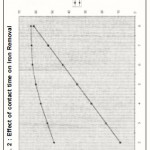 |
Figure. 2 : Effect of contact time on Iron Removal
|
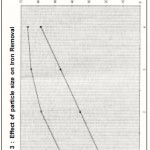 |
Figure 3 : Effect of particle size on Iron Removal |
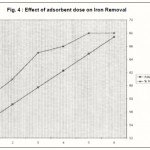 |
Figure 4 : Effect of adsorbent dose on Iron Removal |
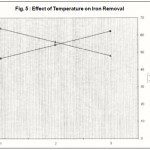 |
Figure 5 : Effect of Temperature on Iron Removal |
Results and Discussion
The effect of particle size of maize husk, contact time, pH and temperature was studied to observe the adsorption of iron and simulate the same at plant level.
Effect of pH
A pH range between 1 and 4 was tried to prevent the precipitation of Fe (II) in basic pH range, 65% of iron uptake by the adsorbent was observed at pH 1.5 as understood from figure -1. With increase in pH, the removal efficiency was decreased which may be due to the hydrolysis of iron. There was no noticeable change in pH before and after the adsorption.
Effect of contact time
The contact period also plays a vital role in ultimate removal of iron. The maximum removal could be effected at 75 minutes. There was no substantial increase in removal efficiency past above time which indicates the attainment of equilibrium time which is evident from figure -2.
Effect of particle size
The effect of particle size on removal of iron from test solution was studied using three different particle sizes (20, 30 and 40 mesh) at fixed pH of 1.5, adsorbent dose of 15g/L, contact time of 75 min and temperature of 30°C. The maximum removal was found to be with mesh size 40. Figure 3 reveal the percentage removal of iron vis-a-vis particle size.
Effect of husk dose
The batch adsorption study was conducted by employing various doses of adsorbent ranging between 5 g/L and 30 g/L at fixed pH, temperature and iron concentration. Figure – 4 reveal that the adsorption increases with increase in adsorbent dose and reaches a plateau at the dose between 25 g/L and 30 mg/L.
Effect of temperature
Adsorption experiments were conducted at three different temperatures viz. 30, 35 and 40°C. With increase in temperature, the adsorption of iron decreases indicating an exothermic nature of the process and might be due to an enhanced escaping tendency of iron from the surface of adsorbent. The adsorption follows the trend 30°C > 35°C > 40°C. Figure -5 show the pattern of graph with respect to this effect.
Conclusion
Maize husk can be a better, cost effective and easily biodegradable substitute to expensive adsorbents for the removal of iron from wastewaters. However, the simulation of these results has not done at the field level to conclude concretely for its efficacy in removing the metal ion in question.
References
- Clement, R.E., Eiceman and G.A. Koester,C.J., Anal. Chem., 67, 221R 91995)
- MacCarthy P., Klusman R.W., Cowling S.W. and Rice J.A., Anal. Chem., 67, 525R (1995)
- Marshall S., Metal and Inorganic Waster Recycling in Cyclopedia Waters, Beta Corporation, Park Ridge, New Jersy.
- Mattson J.S. and Mark H.B., Activated Carbon Surface Chemistry and Adsorption from Aqueous Solution, Marcel Dekker, New York (1971)
- Cheremisinoff P.N. and Ellerbush F., Carbon Adsorption and Handbook, Ann. Arbor Science Publishers, Ann. Arbor, MI (1979)
- Weng, C.H. and Huang C.P., J. Environ. Eng., ASCE, 120, 1470 (1994)
- Singh D.K. and Lal J., Indian J. Env. Health,34, 108 (1992)
- Randall J.M., Reuten F.W. and Waiss, A.C.,J. Appl. Polym. Sci., 19, 1563 (1975)
- Manivasakam N., Industrial Effluents – Origin, Characteristics, Effects and Analysis and Treatment, Sakthi Publications, 385 (1997)
- Standard Methods for the Examination of Water and Wastewater, APHA-AWWAWPCF, Washington, D.C., 20th Ed., (1998)

This work is licensed under a Creative Commons Attribution 4.0 International License.





