Manuscript accepted on : 27-10-2021
Published online on: 29-10-2021
Plagiarism Check: Yes
Reviewed by: Dr. Mohammed Oday
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Ghulam Md Ashraf
Emulgel Approach to Formulation Development: A Review
Brijesh Mahesh Patel , Ashwin Bhanudas Kuchekar
, Ashwin Bhanudas Kuchekar  and Saish Rajendra Pawar
and Saish Rajendra Pawar
School of Pharmacy, Dr. Vishwanath Karad MIT World Peace University, Pune-411038, India.
Corresponding Author E-mail:ashwinkuchekar@yahoo.in
DOI : http://dx.doi.org/10.13005/bbra/2931
ABSTRACT:
Topical drug delivery is the delivery of drugs anywhere in the body through skin, vaginal, ophthalmic and rectal routes. Drugs may be given for localized or systemic effects. Topical formulations with varying physicochemical properties, such as solid, semisolid, or liquid, can be developed. The topical system is created by preparing a drug emulsion and incorporating it into an emulgel. Emulgel is a thermodynamically stable formulation with low interfacial tension that is made by combining a surfactant and a co-surfactant and has several properties such as increased permeability and good thermodynamic stability. Emulgel has a dual control and a sustained release pattern. Emulgel improves bioavailability as well as patient compliance. The pH, viscosity, particle size, zeta potential, drug content, stability study, skin irritation test, and other properties of the prepared formulation are evaluated.
KEYWORDS: Co-surfactant; Emulgel; Gelling agent; Lipophilic; Surfactant
Download this article as:| Copy the following to cite this article: Patel B. M, Kuchekar A. B, Pawar S. R. Emulgel Approach to Formulation Development: A Review. Biosci Biotech Res Asia 2021;18(3). |
| Copy the following to cite this URL: Patel B. M, Kuchekar A. B, Pawar S. R. Emulgel Approach to Formulation Development: A Review. Biosci Biotech Res Asia 2021;18(3). Available from: https://bit.ly/3mrr0do |
Introduction
Topical drug delivery refers to the application of a drug-containing formulation to the skin to treat a cutaneous condition. This system is used when other routes of drug administration (such as oral, sublingual, rectal, and parental) fail, or when a local skin infection, such as a fungal infection, occurs 1. Topical drug administration is a common treatment method for both local and systemic conditions. In the topical delivery system, the drug is absorbed by the skin and reaches the site of action to provide a therapeutic effect. The rate of drug release from a topical preparation is dependent directly on the physiological features of the carrier 2. The primary benefit of a topical delivery system is that it avoids the first-pass metabolism. The term microemulsion is based on particle size. Due to their smaller size, the drug particles can easily diffuse through the skin and reach their site of action. The gel will hold the microemulsion for a long time and will aid in the sustained release of the drug. Various fungal infections are growing nowadays which are a major problem for society. Fungal infections such as Tinea capitis, Tinea pedis and Tinea corporis infect the skin severely. A technique such as emulgel can aid in the easy penetration of the drug into the skin and provide a rapid onset of action.
Physiology of skin
The skin is treated with topical formulations. As a result, a basic understanding of the skin’s physiology and function is essential for developing topical dosage forms. The human skin covers about 2m2 of surface area and provides one-third of systemic circulation through the skin. Per square centimeter of human skin, there are approximately 200-300 sweat ducts and 40-50 hair follicles. The human skin pH ranges between 4.7 to 5.7 3,4.
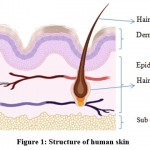 |
Figure 1: Structure of human skin |
Physiological Factors
Lipid Content: Skin is an important water barrier; when the lipid weight in the stratum corneum of skin is minimal, percutaneous penetration increases.
Skin Thickness: The thickness of the skin varies from the epidermal layer to the subcutaneous layer.
The epidermal layer is thick, measuring 100–150 m.
The Density of Sweat Glands.
Hair Follicle Density: The storage capacity of the hair follicle’s infundibulum is approximately ten times that of the stratum corneum.
The pH of Skin: Skin pH increases due to an increase in the secretion of fatty acids and sweat at the surface of the skin.
Skin Temperature: As the temperature increases, the rate of skin permeation increases.
Hydration of Skin: Enhance the permeation of the drug.
Skin Inflammation: As the stratum corneum is disrupted, the permeability increases 5,6.
Table 1: Classification of topical dosage form 7
| Liquid Forms | Solid Forms | Semisolid Forms |
| Syrup | Tablet | Emulgel |
| Solution | Capsules | Creams |
| Emulsion | Powder | Gels |
| Suspension | Dusting Powder | Suppositories |
Emulsion
Emulsions are made by combining two or more liquids that are normally incompatible. In this system, the oil phase is miscible with the aqueous phase using an emulsifying agent. The use of emulsifying agents helps to stabilize emulsions. They are easy to wash off and they also penetrate well 8.
Gel
The word “gel” refers to enhancing the viscosity of liquid preparations without changing other properties. Gels can be used as a thickening agent and also help to improve the homogeneity and consistency of a formulation. This agent is used to create a gel base, which is then mixed with emulsion to create emulgel.
A gel is made up of a polymer that enlarges when exposed to fluid and possibly within its structure. The amount of fluid entrapped in the gel determines its rigidity. These gels are wet and smooth, with the appearance of being solid. These are capable of significant physical deformation, from solid-state to liquid state 9.
Introduction to emulgel
Emulgel is known as an emulsion that has been gelled by using a gelling agent. They can be made either o/w or w/o type. Emulgel is a stable and superior system that incorporates poor water-soluble drugs. In brief, emulgel is a combination of emulsion and gel. Despite the numerous advantages of gels, one significant disadvantage is the delivery of hydrophobic medications. As a result, an emulsion-based solution is being used to overcome this limitation, allowing even hydrophobic therapeutic moieties to benefit from the unique properties of the gel.
Emulgel can deliver both hydrophilic and lipophilic drugs due to the presence of both aqueous and non-aqueous phases. In recent years, they have been used as a control release formulation. These are biphasic systems that have better drug loading capacity and better stability 10,11. Emulgel has several good properties, such as good spreadability, greaseless, thixotropic, good shelf life, odorless, and a pleasant appearance over the conventional topical formulation. Emulgel has both gel and emulsion properties and functions as a dual control release system 12.
Types of emulgel
Microemulsion
Microemulsions are isotropic mixtures of a biphasic o/w systemic stabilized with a surfactant that is thermodynamically stable and optically clear. Droplets vary in size from 10 to 100nm and do not coalesce. It is made up of specific amounts of oil, co-surfactant, surfactant, and water. Microemulsions may have unique properties, including extremely low interfacial tension, a broad interfacial region, and the ability to dissolve both aqueous and oil-soluble compounds. The ingredients in microemulsion could help the drug permeate faster by lowering the stratum corneum’s diffusion barrier.
However, because of their low viscosity, the use of microemulsions in the pharmaceutical industry is limited due to their low skin retention ability. To address this limitation, gelling agents like HPMC K100M, Carbopol 940, and guar gum are added to the microemulsion to form microemulsion-based gels with a viscosity appropriate for topical application 13,14,15.
Nanoemulgel
Nanoemulsion is transparent (translucent) oil-water dispersions that are thermodynamically stable due to surfactant and cosurfactant molecules with a globule size range from 1nm to100 nm. When the emulsion is mixed with gel, the term Nanoemulgel is used. Many drugs have higher transdermal permeation with Nanoemulsion than with traditional formulations such as emulsions and gels. The Nanoemulsion possesses enhanced transdermal and dermal delivery properties in vivo as well as in vitro. Because of its high loading capacity and small globule size, the drug easily penetrates the skin and provides less therapeutic effect in a short period.
Macroemulsion gel
Emulgel with emulsion droplet particle sizes greater than 400nm. They are physically invisible, but under a microscope, the individual droplets can be seen clearly. Macroemulsions are thermodynamically unstable, but surface-active agents can help to stabilize them.
Advantages of emulgel
Using water/oil/water emulsions, hydrophobic drugs can be quickly implemented into the gel base.
Improved stability and load capacity.
Easy for production and a low-cost mechanism.
Avoid sonication.
The first metabolism is avoided.
Avoid gastrointestinal incompatibility.
Target drug delivery on the body.
Improved patient compliance.
Improved patient acceptability and suitability for self-medication.
Ability to easily terminate medication 16.
Disadvantages of emulgel
The drug and/or excipients can lead to skin irritation in people with contact dermatitis.
Some medications have low permeability through the skin.
Possibility of allergenic reactions.
Larger-particle-size drugs are not easily incorporated into the skin 17.
The rationale of emulgel as topical drug delivery
Various semisolids and other preparations are available on the market for restoring the skin’s fundamental role or pharmacologically altering an operation to the underline tissue 18. The formulations, such as lotions, ointments and creams have several drawbacks, including being sticky, having a low spreading coefficient, and having stability issues. Only transparent gels have exposure in pharmaceutical and cosmetic preparations due to overall limitations within the semisolid preparations 19. As a result, an emulsion-based solution is used to address this limitation. Hence, the hydrophobic moiety of the drug should be incorporated and provided through gels. Drug/oil/water emulsions may be used to integrate hydrophobic drugs into emulgel. Since solubility acts as a barrier, most drugs cannot be inserted directly into gel bases, causing problems during drug release. The emulgel system helps to incorporate a hydrophobic drug into the oil phase, after which oily globules are easily dispersed into the aqueous phase, resulting in an oil/water emulsion. The emulsion can be mixed into the gel base. This may result in enhanced drug stability and release over simply incorporating the drug into the gel base 20.
Components of emulgel
Oils are used as an oil phase to prepare an emulsion. Mineral oil and soft or hard paraffin are commonly used, either alone or in combination, in topically applied emulsions.
Example: castor and mineral oils, which have laxative effects, are the most commonly used oils for oral and topical preparations 21,22.
Vehicles
In the emulgel preparation, oily and aqueous vehicles are used, and both hydrophobic and hydrophilic drugs are used.
Examples of vehicles such as alcohol, water, and other aqueous materials are used in aqueous phase emulsions 22.
Emulsifiers
To improve shelf-life stability, an emulsifier is used to increase the emulsification of the preparation. Examples of emulsifying agents are Tween 80, Span80, Tween 20, stearic acid, etc 23.
Gelling agent
Gelling agents are used for preparing gels for any dosage form. It enhances the consistency of any formulation. Some examples of gelling agents are Carbopol 940, Carbopol 934, HPMC-2910, etc 24.
pH adjusting agent
These agents are used to maintain the pH of the formulation. Example: triethylamine, NaOH, etc.
Preparation of emulgel
Step 1: Formulation of gel base
The gel base is formed by dissolving a known quantity of polymer into DDW by mixing at moderate speed using a magnetic stirrer and pH is adjusted to 5-6.5 using Triethanolamine and NaOH 25.
Step 2: Formulation of O/W or W/O type of emulsion
Formulation of Smix in the appropriate ratio using a magnetic stirrer. Add the Smix into the oil phase dropwise under continuous stirring, which gives a clear emulsion 26.
Step 3: Formulation of emulgel
Add the prepared emulsion into gel base dropwise with continuous stirring using a homogenizer to get emulgel27.
Table 2: Marketed formulation of emulgel 28
| Sr. no. | Marketed formulation | API | Manufacturer | Use |
| 1. | Diclobar emulgel | Diclofenac diethyl amine | Barakat Pharma | Anti-inflammatory, analgesic |
| 2. | Voltaren emulgel | Diclofenac diethyl ammonium | Novartis Pharma | Anti-inflammatory |
| 3. | Miconaz-H-emulgel | Miconazole nitrate, Hydrocortisone | Medical union Pharmaceuticals | Topical corticosteroid and antifungal |
| 4. | Diclomax emulgel | Diclofenac sodium | Torrent Pharma | Anti-inflammatory |
| 5. | Levorag emulgel | Hibiscus, licorice, natural extracts | THD Ltd. | Emollient |
Characterization of emulgel
Physical appearance
The color, consistency and homogeneity of the prepared formulation are visually inspected for observations of physical properties 29.
pH measurement
A digital pH meter is used to determine the pH of all prepared emulgel. Calibration of the pH meter is performed before using a standard buffer solution. 1 gm of the formulation is dissolved in distilled water until a uniform suspension is formed and is kept aside for 2 hours. After 2 hours the glass electrode is dipped in the suspension and the pH is measured 30,31.
Rheological study
The viscosity of the prepared formulation is determined at 370C using a cone and plate Brookfield viscometer 32.
Stability study
Stability studies are carried out by inducing stress at different temperatures and humidity (room temperature of 300C±20C, RH of 65%±5% and room temperature of 400C±20C, RH of 75%±5%) using a stability chamber with proper excipient quantity (API-0.1gm, oil-2.5gm, surfactant-6.665gm co-surfactant-13.33gm, double-distilled water 27.15ml).
The study is done for 1 month and observation is done for physical changes such as a change in clarity, observance of turbidity and detection of particle growth 33,34.
Skin irritation test
Skin irritation test is usually done in skin of human volunteers with proper written consent. The prepared formulation is applied to the skin of the hand and observation is done to check for any undesirable effects35.
Zeta potential
The Zeta potential of the emulgel preparation is determined by zetasizer (Malvern Zetasizer) The formulation is placed in a clear, disposable zeta cell, and the result is determined. Before experimenting, cuvettes are washed with methanol and then the sample is placed 36.
Particle size and polydispersity index (PDI)
The globule size of emulgel is measured at 250C by using a zetasizer (Malvern zetasizer instrument, ZS90). The sample is diluted before the experiment37.
Swelling Index
1 mg of gel is placed on porous aluminium foil separately in a 50 ml beaker that contained 10 ml of 0.1 N NaOH. The sample is removed from the beaker at various time intervals and kept in a dry place for some time after it is reweighed [38,39].
Swelling index (SW) = [(Wt.-Wo)/Wo] x a hundred.
Where (SW) %= Equilibrium percentage swelling.
Wo= Original weight of emulgel at zero time where time t,
Wt= Weight of swollen emulgel
Drug Content determination
A spectrophotometer is used to determine the drug concentration in the emulsion. The drug content of an emulsion is determined by sonicating a known amount of emulsion in a solvent (methanol). In a UV/VIS spectrophotometer, absorbance is measured after appropriate dilution 40.
Conclusion
Emulgel is a novel approach that has been proven to be the most convenient, superior, and efficient delivery system. Because of its non-greasy nature and lack of oily bases, it gives gel-like properties and gives excellent drug release when compared to conventional topical delivery systems. Emulgel has a high drug loading capacity and is effective in drug delivery at the target site. Penetration of a drug through the skin is effective due to its small particle size. Emulgel is formed by incorporating emulsion into the gel base and provides a dual control release effect. The emulgel technique helps to solve different problems, such as creaming, phase separation and its stability improves. Hydrophobic drugs can be delivered with the help of emulgel and they can be incorporated into the oil phase of the emulsion and combined with gel. This technique improves patient compliance and increases the bioavailability of the drug in specific areas.
References
- Das S K, Khanum A, Ghosh A. Microemulsion based gel Technique- A Novel Approach for Sustained Delivery to Treat Fungal Infection. Indo American Journal of Pharmaceutical Research. 2019; 8 (2): 1958.
- Kumar N, Saxena C, A Novel Approach for Topical Drug Delivery System -Emulgel Trends in Pharmaceutical and Nanotechnology. 2019, 1 (2), 27-28.
- Mathew N J, Mathew F, Eldhouse M P, “Emulgel- A Formulation for Topical Delivery of Hydrophobic Drugs, International Journal of Universal Pharmacy and BioScience, 2016,102-114.
- Ashara K C, Paun J S, Soniwala M M, Chavada J R, Mori N M, “Micro- Emulgel based Emulgel: A Novel Topical Drug Delivery System, Asian Pacific Journal of Tropical Disease 2014, S28.
CrossRef - Panwar A S, Jain D K. Emulgel; A Review. Asian Journal of Pharmacy and Life Science. 2011;1:336-337.
- Sah S K, Badola A, Nayak B K. Emulgel: Magnifying the application of topical drug delivery. Indian Journal of Pharmaceutical and Biological Research. 2017; 5(1):25-33.
CrossRef - Arora R, Khan R, Ojha A, Upadhyaya K, Chopra H. Emulgel- A Novel Approach for Hydrophobic Drugs. International Journal of Pharmacy and Biological Science. 2017; 7(3):43-45.
CrossRef - Yogi J, Dabhi V, Chaudhary S, Shah H, Sanghvi K. Microemulsion as Advance Topical Drug Delivery: A Review. International Journal of Pharmaceutical Research and Bioscience. 2015; 4(1):321-322.
CrossRef - Sreevidya V S. An Overview on Emulgel.International Journal of Pharmaceutical and Phytopharmacological Research. 2019; (9)1:93-94.
CrossRef - Light k, Karboune S. Emulsion, hydrogel and Emulgel system and novel application in cannabinoid delivery: a review. Taylor & Francis Group. 2021; 22:1-31.
CrossRef - Mohammed Haneefa P K. Emulgel: An Advance Review. Journal of Pharmaceutical Science and Research, 2013;5 (12): 254 – 258.
CrossRef - Satya Lakshmi S, Divya R, Srinivasa Rao Y, Kamala Kumari PV, Deepthi K. Emulgel-Novel Trend in Topical Drug Delivery System – Review Article. Research J. Pharm. and Tech. 2021; 14 (5): 2903-2906.
CrossRef - Sharma A K, Tarun Garg, Goyal A K, Rath G. Role of microemulsion in advance drug delivery. Informa healthcare. 2014 Dec; 4: 1177-1185.
- Anand K, Ray S, Rahman M, Shaharya M A, Bhowmik R, Bera R. Nano-Emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications. Recent Patents on Anti-Infective Drug Discovery. 2019; 14 (1): 16-35.
CrossRef - Hyma P, Jahan N, Raheemunissa, Sreelekha G, Babu K. Emulgel: A Review. International Journal of Pharmaceutical Archive. 2014 May; 2(3): 459-467.
- Yadav S K, Mishra M k, Tiwari A, Shukla A, ‘Emulgel: A New Approach for Enhanced Topical Drug Delivery, 2017, 9 (1), 15.
CrossRef - Jain S K, Bajapi P, Modi S K, Gupta P, ‘A Review on Emulgel, as a Novel Trend in Topical Drug Delivery’, Recent Trends in Pharmaceutical Sciences and Research, MAT Journal, 2019, 1 (2), 31-21.
- Singh R P, Parpani S, Narke R, Chavan N R, ‘Emulgel: A Recent Approach for Topical Drug delivery System’, Asian Journal of Pharmaceutical Research and Development, 2014, 2, 114.
- Khare S, Abyankar S. Kuchekar A, Gawade A, A Mini Review – Pharmaceutical Creams, Sch Acad J Pharm, 2021, 10 (04), 60-62.
- Dhawas V, Dhabarde D, Patil S, Emulgel: A Comprehensive Review for Novel Topical Drug Delivery System, International Journal of Recent Scientific Research, 2020, 11 (04), 38135-38136.
- Sanjay, Jain BD, Padsalg A, Patel K, Mokale V, Formulation, development, and evaluation of Fluconazole gel in various polymer bases, Asian. J. Pharma, 2007; (1); 63-69.
- Charyulu N R, et al, Emulgel: A Boon for Enhanced Topical Drug Delivery, J Young Pharm, 2021; 13 (1): 76-79.
CrossRef - Gousia B S et al, A Review on Emulgels-A Novel Approach for Topical Drug Delivery, Asian Journal of Pharmaceutical Research and Development. 2019; 7(2): 70-77.
CrossRef - Kegade P et al, Emulgel: In Treatment of Periodontitis, World Journal of Advance Healthcare Research, 2020, 4 (5), 71-75.
CrossRef - Kumar D, Singh J, Antil M, Kumar V, Emulgel-Novel Topical Drug Delivery System–A Comprehensive Review, International Journal of Pharmaceutical Science and Research, 2016, 7 (12), 4735.
- Manmode P D et al, Formulation and Evaluation of Flurbiprofen Emulgel by Using Natural Permeation Enhancers, 2021,10 (1), 827-828.
- Baibhav J et al, Emulgel: A Comprehensive Review on the Recent Advances in Topical Drug Delivery, International Research Journal of Pharmacy, 2011, 2 (11),66-70.
- Ojha A, Ojha M, Satheesh Madhav N V, ‘Recent Advancement in Emulgel: A Novel Approach for Topical Drug Delivery, International Journal of Advances in Pharmaceutics, 2017, 21.
- Ambhore N P, Dandagi P M, Gadad A P, Mandora P, ‘Formulation and Characterization of Tapentadol Loaded Emulgel for Topical Application, Indian Journal of Pharmaceutical Education and Research, 2017, 51 (4), 527-529.
CrossRef - Rode R J et al, A Comprehensive review on Emulgel: A New Approach for Enhanced Topical Drug Delivery, International Journal of Modern Pharmaceutical Research, 2021, 5 (3), 222-233.
- Goyani M et al, Formulation and Evaluation of Topical Emulgel of Antiacne Agent, International Journal of Advanced Research and Review, 2018, 3 (7), 52-68.
- Jain A, Gautam S P, Gupta Y, Khambete H, Jain S, Development and characterization of ketoconazole emulgel for topical drug delivery, Pelagia Research Library Der, 2010, 1 (3): 221-231.
- Anand K et al, Nano-emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications, Recent Patents on Anti-Infective Drug Discovery, 2019, 14, 16-35.
CrossRef - Jivani M N, Patel C P, Prajapat B G, Nanoemulgel Innovative Approach for Topical Gel Based Formulation, Research and Reviews on Healthcare: Open Access Journal, 2018, 18-22.
- Pawbake G R, Shirolkar S V, Microemulgel: A Promising Approach to Improve the Therapeutic Efficacy of Drug, Journal of Critical Reviews, 2020, 7 (14), 1138-1142.
- Suman D, Sangeeta, Beena K, Emulgel for topical drug delivery: A novel approach, GSC Biological, and Pharmaceutical Sciences, 2020, 11 (03), 104-114.
CrossRef - Chaitali J, Vaishali K, Santosh P, “Formulation and Evaluation of Antifungal Non-aqueous Microemulsion for Topical Drug Delivery of Griseofulvin, Inventi Impact: Pharm Tech, 2015, (1), 38.
- Iradhati A H, Jufri M, “Formulation and Physical Stability Test of Griseofulvin Microemulsion gel, International Journal of Applied Pharmaceuticals, 2017, 9, 25-26.
CrossRef - Shehata TM, Nair AB, Al-Dhubiab BE, Shah J, Jacob S, Alhaider IA, Attimarad M, Elsewedy HS, Ibrahim MM. Vesicular Emulgel Based System for Transdermal Delivery of Insulin: Factorial Design and in Vivo Evaluation. Applied Sciences. 2020; 10 (15):5341.
CrossRef - Sushma G et al, Emulgels- A Novel Approach for Topical Drug Delivery, International Journal of Pharmaceutical Sciences Review and Research, 2021, 67 (1), 142-147.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





