Manuscript accepted on : 22-May-2019
Published online on: 25-05-2019
Plagiarism Check: Yes
Reviewed by: Ali Elshafei
Second Review by: Wael Mahmoud Aboulthana
Metal Nanodelivery Systems for Improved Efficacy of Herbal Drugs
Sonu Ambwani*1, Roopali Tandon2 and Tanuj Kumar Ambwani3
1Department of Molecular Biology and Genetic Engineering, College of Basic Sciences and Humanities, GB Pant University of Agriculture and Technology, Pantnagar 263145, Uttarakhand, India.
2Department of Chemistry, Bareilly College, MJP Rohilkhand University, Bareilly, Uttar Pradesh, India.
3Department of Veterinary Physiology and Biochemistry, College of Veterinary and Animal Sciences, GB Pant University of Agriculture and Technology, Pantnagar 263145, Uttarakhand, India.
Corresponding Author E-mail: ambwani_sonu@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2741
ABSTRACT: Herbal drugs have been used since ancient times in various parts of the world. These have wide acceptability due to their time-tested therapeutic values and minimal side effects in contrast to modern allopathic medicines. Mostly, the herbal drugs are either in dried powder form or in crude extract form prepared in different solvent systems. These preparations generally need large dose administration and also could be less effective in the form of conventional formulations. Moreover, these herbal formulations cannot be targeted to specific tissue in case of different chronic diseases. Oral consumed herbal formulations display reduced bioavailability as these are subjected to adverse pH, enzymatic degradation and ultimately poor gut absorption. Constraints associated with conventional phytopharmaceuticals have been improved by designing and using “Nano Delivery Systems” (NDS). The foremost aim of NDS is to provide sustained drug release, site-specific action, and improved patient’s compliance. Nanometal based herbal drugs can be used for targeted drug delivery in the body which improves their safety, effectiveness and reduces need of frequent large doses. Metal Nanocarriers loaded with herbal drugs can carry the optimal amount of the drug to their site of action avoiding different obstructions such as low pH in the stomach, metabolism by liver so that the drug can circulate into the blood for a longer period of time. Herbal drugs with NDS thus would be helpful in enhancing their efficacy.
KEYWORDS: Core Shell Nanoparticles; Herbal Drug; Metal Nanodelivery Systems
Download this article as:| Copy the following to cite this article: Ambwani S, Tandon R, Ambwani T. K. Metal Nanodelivery Systems for Improved Efficacy of Herbal Drugs. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Ambwani S, Tandon R, Ambwani T. K. Metal Nanodelivery Systems for Improved Efficacy of Herbal Drugs. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2K1NU8h |
Introduction
From past two decades, nanotechnology is exploited for efficacious drug delivery and tissue-specific targeting of drug (Kumar et al., 2015). Improved drug delivery techniques help in minimizing toxic effects and achieving enhanced effectiveness which is beneficial for the patients. In recent past, there has been a renewed public interest in natural remedies both in developing and developed countries. As per World Health Organization (WHO) “herbal medicines as finished, labeled medicinal products that contain active ingredients, aerial or underground parts of the plant or other plant material or combinations”. According to WHO reports, 80% of the populations from developing countries exploit herbal medicines for their primary health care needs (Yadav et al., 2014). Herbal ‘renaissance’ is happening all over the world due to rising concern over the safety of modern allopathic medicines. Even the genesis of modern medicine is from traditional therapeutic systems (Patwardhan et al., 2004). Plants and natural products have been used since long for curative/ healing purposes in different cultures like China, Egypt, Africa, America and India. Herbal medicine, also known as “herbalism” or “botanical medicine” is a medical system based on the use of plants or plant extracts that may be taken orally or applied to the skin (Griggs, 1982). In spite of criticisms regarding certain features of herbal medicine, many pharmacologists today, recognize the potential scientific rationale of biological effects produced by these phytomedicines (Sharma et al., 2011).
Though herbal medicines are considered as dependable and affordable therapeutics, however, some problems are associated with them viz. rapid release of the herbal drug, unknown toxicity, low solubility, poor bioavailability and oral absorption, etc. (Thillaivanan & Samraj, 2014). Besides the general notion that “the herbal drugs are safe”, many pharmaco-vigilance studies have suggested that these natural drugs have frequently unknown active ingredients and thus their standardization and quality control are not an easy task (Ekor, 2013; Mathur, 2016). Delivery of herbal medicines also requires an improved delivery system for their sustained release and targeted delivery for enhanced patient compliance (Goyal et al., 2011). NDS is a new concept of drug delivery that helps to overcome the limitation of traditional drug delivery systems. NDS, when employed for herbal medicine, may be helpful in enhancing the efficacy and lowering their side effects (Ansari et al., 2012). In novel phytoformulations, various nano-delivery vehicles, viz., liposomes, polymeric nanoparticles, nanoemulsion, nanospheres, solid lipid nanoparticles (SLNs), are used in which phytoconstituents can be incorporated and these nanosized herbal formulations not only help in sustained release of the drug but can also eliminate the inadequacies of conventional herbal formulations (Elmowafy et al., 2013; Priprem et al., 2015). Present communication explicates upon various metal nano delivery systems that could be used for herbal formulations and thus could be helpful in improving therapeutic efficacy of phytomedicines.
Herbal Drugs
Herbal drugs based curative system is one of the oldest forms of health care system. Plant based formulations may prove exceedingly important for the healthcare of a person (Mathur, 2016). A vast increase in global human population, inadequate accessibility of expensive allopathic drugs coupled with unwanted side effects and problems like multidrug resistance in dangerous pathogens have led way to the development of alternative plant-based natural medicines for a broad range of diseases (Greenwell & Rahman, 2015). Phytomedicine or phytopharmaceutical, is a complex mixture derived from plant sources that is used as a medicine or drug. Approximately 50% of the functional drugs are prepared from natural resources (Kingston, 2011).
Advantages and Disadvantages of Herbal Drugs
Herbal formulations are prepared from natural ingredients and are available at affordable cost. Herbal drugs are time tested, recognized for their therapeutic potential with little or no side effects as compared to allopathic medicines (Mahima et al., 2012). Herbal drugs can be exploited for treating different pathological conditions. The therapeutic potential of herbal drugs are due to the presence of various secondary metabolites like phenolics, terpenoids (sapogenin), alkaloids, steroids etc., in them (Mathur, 2016).
Most of the traditional herbal preparations are crude formulations with low solubility because of the presence of hydrophobic phytochemicals. Major constraint that limits the use of herbal drugs is their low efficacy because of their poor solubility, bioavailability and oral administration (Jantarat, 2013; Figure 1). Traditional herbal preparations are not favored for development of new drug formulations due to lack of chemical characterization, standardization, validation and scientific rationalization of their medicinal potential (Ekor, 2013; Thillaivanan & Samraj, 2014). Mostly, traditional herbal preparations are given through oral route and thus a low quantity of drug reaches to the site resulting in low therapeutic value as large amount of these drugs get wasted due to their distribution throughout the body depending on physicochemical and biochemical properties. Many herbal constituents may get degraded in the stomach due to the acidic environment while some others may be metabolized by liver and thus insufficient amount of the phyto-compounds reach the blood (Yadav et al., 2011; Ansari et al., 2012). Either poor or no therapeutic impact may be observed if insufficient quantity of the drug is administered instead of the optimal quantity/ dose (minimum effective dose level). Some phytochemicals have low solubility and bioavailability and thus exhibit narrow therapeutic index. Researchers are constantly trying to enhance the therapeutic efficacy and better patient compliance by specific targeting of the drug and its controlled release (Park, 2014).
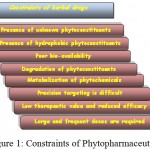 |
Figure 1: Constraints of Phytopharmaceuticals.
|
Due to various limitations of herbal drugs and low efficacy of the treatment of relentless diseases, multidisciplinary strategies are put forward for targeted delivery to improve their pharmacokinetics, pharmacodynamics, bio-recognition and efficacy. Such innovative delivery methods require interdisciplinary inputs of polymer chemistry, pharmacology, nanotechnology, bioconjugate chemistry, etc (Charman et al., 1999).
Nano Delivery Systems for Herbal Drugs
Mostly “Novel Drug Delivery Systems” (NDDS) are employed in case of allopathic medicines especially for cancer theranostics. However, NDDS can be effectively used for herbal drugs as well which are fast becoming an important form of alternative or complementary therapy. Salient aspects of herbal therapy that need attention and improvement are- enhanced component solubility, better bioavailability, enhanced absorbency, reduction in doses and overall better compliance (Ansari et al., 2012; Figure 2). Suitable routes of drug delivery are employed to cross physical barriers, viz. blood-brain barrier (BBB) (Kumar et al., 2015).
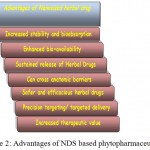 |
Figure 2: Advantages of NDS based phytopharmaceuticals.
|
Process of administering pharmaceutical molecules by an appropriate route in the patient can affect the therapeutic efficacy of the drug as well. Some of the common routes of drug delivery are per-oral (through the mouth), topical (skin), transmucosal (nasal, buccal, sublingual, vaginal, ocular and rectal) and inhalation routes. Nano delivery systems (NDS) includes different approaches for the delivery of pharmaceuticals in the body which may either require its specific targeting or it might require systemic pharmacokinetics to get desired therapeutic efficacy of the drug. NDS are advance delivery approaches to enhance drug effectiveness by targeting the drug to the desired site, by controlling drug release to provide sustained curative effect and offer better safety (Nagavarma et al., 2012). NDS includes carrier based drug delivery system (liposomes, niosomes, microspheres, resealed erythrocytes as drug carriers), trans-dermal Delivery Systems (sonophoresis), mucoadhesive delivery systems, supramolecular delivery systems and variable release delivery systems (osmotic pump, nanoencapsulation), etc (Figure 3). Development of NDS for herbal formulations is a tricky task because of the presence of chemically diverse phytoconstituents in herbal formulations (Nagavarma et al., 2012).
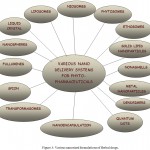 |
Figure 3: Various nanosized formulations of Herbal drugs.
|
To enhance selectivity, solubility, safety and effectiveness of herbal drugs NDS can be used. Nanosized herbal drug has enhanced surface area and thus has faster distribution in the blood and reduced toxicity with better therapeutic efficacy. Improved permeation and retention of nanoparticles (NPs) can also help to cross BBB (Kumar et al., 2015; Chakraborty et al., 2016). Therefore, amalgamation of NDS in the traditional remedies is of vital importance for treatment of many chronic ailments like asthma, cancer, etc. (Bonifacio et al., 2014; Aqil et al., 2013; Sharma & Singh, 2014; Gunasekaran et al., 2014; Mathur, 2016; Ambwani et al., 2018b). For example flavonoids exhibit poor stability, bioavailability, and bioefficacy if administered through oral route (Manach et al., 2005) and thus, different bioactivities obtained in experimental in vitro conditions are not replicated in in vivo conditions. Flavonoids displayed improved stability and absorption when administered through NDS (Dube et al., 2010). It is further reported that nano-based flavonoids possess enhanced surface area, better stability and bioavailability due to receptor-mediated phagocytosis and endocytosis by specific cells (Tan et al., 2012). NDS also mediate controlled discharge of encapsulated flavonoids. Bilia et al. (2014b) concluded that nanocarriers made of approved molecules referred to as “Generally Recognized as Safe” (GRAS), may be used for development of efficacious preparations of herbal functional foods, dietary supplements and therapeutics. Different types of nanosized herbal formulations can be prepared to achieve the enhanced therapeutic potential of phytomedicines.
Metal Nanoparticles Based Drug Delivery
Different metal nanoparticles (MNPs) based herbal formulations are reported to be more efficacious. Most extensively used MNPs for various biomedical applications are gold, silver, iron and copper, however other MNPs, such as, zinc oxide, titanium oxide, platinum, selenium, gadolinium, palladium, cerium dioxide are also explored. “Ayurveda” is an ancient Indian health care system that is based on plants and herbo-mineral preparations. Biologically prepared metal nanoparticles known as “Bhasma” are used for the treatment of various diseases/ disorders in Ayurveda (Galib et al., 2011; Pal et al., 2014). Nano-forms of certain metals are commonly used in Ayurveda: gold (Au), silver (Ag), copper (Cu), iron (Fe), lead (Pb), tin (Sn) and zinc (Zn) (Sarkar et al., 2010). For development of such formulations, biocompatibility and therapeutic efficacy of phytochemicals found in the plants play central role in choosing various herbs. Herbo-mineral formulations may exhibit enhanced therapeutic properties because of their physiological compatibility, insignificant toxicity and natural origin. Tremendous applications of such nanomaterials based herbal preparations in the field of biomedicine have led to an innovative concept of ‘Herbo-nanoceuticals’ (Gomes et al., 2014).
Many studies have shown that during the processing of ayurvedic metal based formulations, size of the metal particles come down to nanometer range (Pavani et al., 2013). Gold is used as Swarna Bhasma (gold ash) in different conventional Indian ayurvedic preparations and has been characterized to possess 56-57 nm size and spherical shape. Mercury-based ayurvedic preparations have crystalline mercuric sulfide of 25-50 nm size range (Pal et al., 2014). Pavani et al. (2013) reported a modified method of ‘bhasmikaran’ (method of preparing bhasmas) for preparation of iron oxide nanoparticles which was based on green synthesis. X-ray diffraction and transmission electron microscopy (TEM) analyses demonstrated that during ayurvedic ‘bhasmikaran’ method MNPs are formed. MNPs along with different phytochemicals show enhanced absorption and even tissue-specific targeting of therapeutic ingredients inside the body. Because of their small size, these preparations are said to be more effective. More over these naturally synthesized MNPs do not cause toxicity in the body (Paul and Chugh, 2011). Mukundan et al. (2015) reported green synthesis of silver nanoparticles using leaves extract of Bauhinia tomentosa Linn and explored its in vitro anticancer potential.
Green synthesis of MNPs could be carried out through many natural compounds viz. vitamins, carbohydrates (sugars), phytochemicals present in plant extracts, microbes, biodegradable polymers etc. Plant extracts are used for the commercial preparation of MNPs because of the presence of phytocompounds having strong reducing potential (Iravani, 2011; Shah et al., 2015). Polyphenols with their hydroxyl side chains are the main phytocompounds present in the plant extracts that possess the ability to act as reducing agent. Polyphenols can act as capping and stabilizing agents for MNPs formation. Gold (Au) NPs have better biocompatibility and thus are the most used MNPs in the area of biomedical sciences (Bhattacharya & Mukherjee, 2008; Ambwani et al, 2018a). Geetha et al. (2013) reported a quick, economical and single-step method for formation of AuNPs with the help of flower extract of Couroupita guianensis. AuNPs green synthesis using glucoxylans of Mimosa pudica seeds was carried out without using any chemical stabilizing agent (Iram et al., 2014). Rao et al. (2016) reviewed different medicinal plants and their active compounds along with green-synthesized MNPs from medicinal plants in context to their anticancer efficacy. Plant extracts based MNPs displayed enhanced tumor specificity, promising cytotoxicity and insignificant toxicity for healthy cells. The cytotoxicity of MNPs may be due to their large surface area that leads to efficient drug delivery while some MNPs have been shown to possess anticancer potential (Ambwani et al., 2016; Datta et al., 2018). However, there is need to conduct in vivo studies to validate the actual efficiency of herbal MNPs based drugs (Rao et al., 2016). Several types of MNPs such as quantum dots, metal oxides and pure metal NPs are reported to be useful for biomedical usage (Bonfacio et al., 2014, Sharma & Singh, 2014; Ambwani et al., 2015). Many researchers have reported different microorganisms and plant extracts strategies for green synthesis of MNPs which are said to be eco-friendly, economical and non-toxic (Makarov et al., 2014). However, there is need to address certain problems associated with MNPs and ensure their biosafety before proposing their biomedical usage and their commercialization. Though many reports are available underlining usage of MNPs for different theranostic and biomedical applications, however, their potential side effects in the patients and environment are yet to be thoroughly explored (Krug & Wick, 2011). Contradictory biosafety considerations are reported pertaining to MNPs in different studies (Tsoi et al., 2012; Edmundson et al., 2014; Ambwani et al., 2015).
Nanoshells
Nanoshells are also known as core–shells which are few nanometers (1-20 nm) in size. Nanoshells have sphere-shaped cores (concentric particles) prepared of a particular compound which is surrounded by an outer coating or shell made up of a different compound (Kalele et al., 2006). By changing core-to-shell ratio or constituent compounds of nanoshells, the properties of these core shells can be changed. Semiconductor materials, insulators or metals may be used for the preparation of core shells. Dielectric compounds like silica and polystyrene with high stability are typically employed for the preparation of cores (Ansari et al., 2012; Sachan & Gupta, 2015). A novel type of sphere shaped composite metal nanoshells has been prepared in which dielectric material is used for core preparation that is surrounded by a thin metallic shell usually made up of gold. Nanoshells have numerous usages in the biomedical area especially in drug delivery and in vivo imaging-based diagnostic strategies as they possess unique optical and chemical attributes. Nanoshells can absorb heat and when it is illuminated by suitable wavelength then it can transmit the heat to the local environment that leads to discharge of the cargo compound by breaking down the core-shell assembly (Kalele et al., 2006). IN core shell delivery systems, drug molecules can either be encapsulated by the shell or can be adsorbed on the surface of the shell with the help of specific functional group or could be stabilized by electrostatic bonding in the nanoshell based drug delivery systems. Upon coming in contact with the biological systems, these drug-loaded nanoshells release the drug. Monoclonal antibodies specific for a diseased tissues or tumors can be attached on the surface of nanoshells in case of targeted drug delivery and in vivo diagnostic (imaging) approaches which make these core shells to be efficacious for cancer theranostics (Mamillapalli et al., 2016; Singhana et al., 2014).
Gold nanoshells (AuNSs) are widely investigated as targeted nanovehicles for drug carriers that could be exploited for theranostic purposes. These have potential applicability in the field of oncology including photothermal ablation, hyperthermia, drug delivery and diagnostic imaging. AuNSs are of paramount importance because of their biocompatibility, non-toxicity, surface plasmon resonance, insignificant immunogenicity and surface functionalization. AuNSs may be used for accurate and targeted drug delivery in the specific cells along with radiotherapy. Singhana et al. (2014) explored NIR activated AuNSs based drug delivery systems and cancer theranostics.
Quantum Dots (QDs)
Quantum dots are tiny microscopic metal or semiconductor particles that hold a certain number of electrons and, upon light excitation can emit light in all the colors of a rainbow depending on their size. QDs when tagged with specific molecules are capable of identifying the specific tissues. Various surface modifications can prevent aggregate formation, reduction in non-specific attachment and facilitate tissue specific targeting in case of in vivo imaging approaches. Functionalization of QDs can be done by modifying protecting ligands (Mulder et al., 2009) and addition of targeting groups viz. lipoproteins and peptides. Near-IR QDs tagged with a monoclonal antibody against tumour cells are common QDs used for targeted delivery. Development in this area is being appraised by many researchers (Papagiannaros et al., 2010). For various bio-applications, surface functionalization of QDs can be carried out (Juzenas et al., 2008). Singh et al. (2017) reported curcumin QDs mediated degradation of bacterial biofilms. Applications of QDs in photodynamic and radiation therapies for cancer have been reviewed extensively (Tan & Zhang, 2005). Zhao et al. (2012) reported prompt programme cell death in the hepatic cells due to exposure of eta-Cyclodextrin/glycyrrhizic acid functionalized QDs.
Covalent or non-covalent binding of specific molecules like antibodies, peptides, and aptamers onto QDs, could be used for targeted delivery of hydrophilic therapeutic molecules like small interfering RNA (siRNA) and antisense oligodeoxynucleotide (ODN). These nanocomposites are like ‘magic bullets’, which can bind to specific cells/ tissues and cure it by releasing therapeutics on the site. QD based nano vehicles can be tracked in real-time due to emission of signals which make them suitable for in vivo imaging, localization of metastasis and cancer diagnostics (Belletti et al., 2017; Ambwani et al., 2015). Belletti et al. (2017) reported that the single-emulsion process for curcumin loading into NPs with encapsulation efficiency about 35%. Nanonization of phytopharmaceuticals displayed increased surface area to volume ratio, improved stability, solubility and bioavailability, reticuloendothelial system (RES) uptake, better penetrability and retention, site-specific targeting, controlled release, etc (Goyal et al., 2011; Naghsh, 2015).
Biosafety of Nanosized Herbal Formulations
Even though nano-delivery based herbal preparations have shown their potential applicability by overcoming the inadequacies of conventional herbal formulations, however, their safety concerns should not be ignored (Ambwani et al., 2015). For exploitation of nanotechnology in the area of biomedicine it is essential to ensure their biocompatibility and safety as well as to rule out any probability of undesired toxic effects of nanoformulations to human/ environment. In case of herbal remedies, nano-delivery approaches can be fruitful in enhancing the therapeutic ratio or index by increasing the gap between minimal dose required to exhibit the curative effects and minimum toxic level dose that display unwanted side effects. In spite of the potential benefits, there is a need for intensive toxicological examination of different nano-herbal formulations. The alteration in the physico- chemical, optical, structural attributes of engineered nanomaterials may lead to numerous unpredicted interactions with different biological components which can lead to toxic side effects (Chan et al., 2010).
At present, still there is a lack of methodical toxicological profiling of nanomaterials and nanomedicines, with insufficient and sometimes contradictory safety and toxicity data available. Voigt et al. (2014) reported Polybutylcyanoacrylate NPs (PNPs) to be non-toxic and thus could be useful in the field of nanomedicine. However, Sadat et al. (2016) gave a concise summary of the alterations in various attributes of PNPs due to a different size and surface charge and what problems could arise during in vivo experimentations. Some studies have been conducted to exhibit either in vitro or in vivo toxic effects of various NPs (Wang et al., 2013; Ambwani et al., 2016, Datta et al., 2018; Ambwani et al., 2018). Several contradictory in vivo/ in vitro reports are available regarding nanomaterials toxicity. Metal oxides are reported to exhibit enhanced oxidative stress (Bonner, 2007). Foremost reason of formation of reactive oxygen species (ROS) due to NPs exposure is presence of pro-oxidant functional groups on the surface of NPs, active redox reactions happening on the surface of NPs especially in case of MNPs and different interactions between cells and MNPs. Entry of NPs inside the cells can activate various immune cells like macrophages and neutrophils that are potent phagocytes and generate ROS/reactive nitrogen species (RNS) once activated (Ambwani et al., 2015, Datta et al., 2018; Ambwani et al., 2018). NPs toxicity could be due to their dose and route of exposure as well as different physic-chemical attributes of NPs. Another important feature in toxicity profiling of NPs is getting conflicting results during in vitro and in vivo toxicity analysis of same NP. Presently there are no guidelines, regulations or standard procedures in place for methodical toxicological analysis of NPs. The imprecise and ambiguous conclusions associated to NPs toxicity shed disbelief on the potential of nanomedicine and cause pointless apprehensions in the public mindset (Yildirimer et al., 2011). However, suitable nano-herbal formulations can be prepared after thorough analysis of various interactions between engineered NPs and target cells, resultant toxicity its scientific rationale.
Conclusion
Treatment of any disease is based either on development of better drugs or by enhancing efficacy of existing drugs. For better safety and efficacy of existing formulations, different nano-based drug delivery approaches have attracted the attention of the researchers. Approaches for drug delivery have undergone many changes involving engineered materials with novel physical or chemical effects. The conventional drug delivery methods have several constraints in development of efficacious herbal drugs because oral or parenteral herbal formulations are reported to be inefficient due to several drawbacks involved. Thus it could be concluded that usage of NDS could be of paramount importance in designing herbal formulations. MNPs based herbal drug delivery systems have displayed tremendous potential for various diseases and disorders especially for cancer theranostics. MNPs based herbal formulations have shown enhanced uptake, bioavailability and accurate delivery.
Globally, herbal and natural products are gaining pace. Thus amalgamation of “herboceuticals” with nanotechnology would improve their therapeutic potential for treatment of various chronic diseases. Nanosized herbal drugs are studied in different biomedical research institutions. The significant attribute needed in herbal drugs are their site-directed sustained delivery with enhanced curative potential, patient compliance and without unwanted toxic side effects and hypersensitive reactions, etc. Hence, NDS based herbal formulations will boost their ability to treat different chronic health conditions and impart tremendous fitness benefits. This type of multidisciplinary research including traditional herbal therapeutics together with novel modern drug delivery systems has given way to the development of attractive nanosized herbal drugs as future phytopharmaceuticals that would prove to be of paramount value for enhancing the health of people.
References
- Ambwani S., Kakade D. P., Arora S., Ambwani K. Nonlinear response of Gold nanoparticles pertaining to immunotoxicity in chicken lymphocytes culture system. Research Journal of Biotechnology, 2018a;13(11): 64-73.
- Ambwani S., Tandon R., Ambwani K., Malik Y. S. Current knowledge on nanodelivery systems and their beneficial applications in enhancing the efficacy of herbal drugs. Journal of Experimental Biology and Agricultural Science, 2018b; 6(1): 87-107.
- Ambwani S., Tandon R., Gupta A., Ambwani T. K., Chauhan R. S. Nanoparticles: Utility, immuno-toxicology and ethical issues. Journal of Immunology and Immunopathology, 2015;17: 68-78.
CrossRef - Ambwani S., Tandon R., Gupta A., Ambwani K., Chauhan R. S. Nanoparticles: Utility, Immuno-Toxicology and Ethical Issues. Journal of Immunology and Immunopathology, 2015; 17 (2): 68-78.
CrossRef - Ambwani,Kakade D. P., Kandpal D., Arora S., Ambwani T. K. Cytotoxic effects of gold nanoparticles exposure employing in vitro animal cell culture system as part of nanobiosafety. AIP Conference Proceedings 1724, 020091 2016; doi: http://dx.doi.org/10.1063/1.4945211.
CrossRef - Ansari S. H., Islam F., Sameem M. Influence of nanotechnology on herbal drugs: A Review. Journal of Advanced Pharmaceutical Technology & Research, 2012; 3: 142-146.
CrossRef - Aqil F, Munagala R, Jeyabalan J, Vadhanam MV. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Letters 2013; 334(1): 133–141.
CrossRef - Bhattacharya R., Mukherjee P. Biological properties of “naked” metal nanoparticles. Advanced Drug Delivery Reviews 2008;60: 1289-1306.
CrossRef - Bilia A. R., Isacchi B., Righeschi C., Guccione C., Bergonzi M. C. Flavonoids Loaded in Nanocarriers: An Opportunity to Increase Oral Bioavailability and Bioefficacy. Food and Nutrition Sciences, 2014b; 5: 1212-1227.
CrossRef - Bonifacio B. V., d Silva P. B., Ramos S., Silveira Negri K. M., Bauab T. M., Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: A review. International Journal of Nanomedicine, 2014; 1-15.
CrossRef - Bonner C. Lung fibrotic responses to particle exposure. Toxicologic Pathology, 2007; 35(1): 148–153.
CrossRef - Chakraborty K., Shivakumar A., Ramachandran S. Nano-technology in herbal medicines: A review. International Journal of Herbal Medicine, 2016; 4(3): 21-27.
CrossRef - Chan, E.-S., Yim Z.-H., Phan S.-H., Mansa R.F., Ravindra, P. Encapsulation of herbal aqueous extract through absorption with ca-alginate hydrogel beads. Food Bioprod Process., 2010; 88: 195-201.
CrossRef - Charman W. N., Chan H. K., Finnin B. C., Charman S. A. Drug delivery: A key factor in realising the full therapeutic potential of drugs.Drug Development Research, 1999; 46:316–27.
CrossRef - Datta P. K., Arora S., Ambwani S. Cytotoxic effect of silver nanoparticles in cancerous HeLa cells due to enhanced oxidative stress. Research Journal of Biotechnology, 2018; 13(2): 68-74.
- Dube A., Nicolazzo J. A., Larson I. Chitosan Nanoparticles Enhance the Intestinal Absorption of the Green Tea Catechins (+)-Catechin and (−)-Epigallocatechin Gallate. European Journal of Pharmaceutical Sciences, 2010; 41: 219- 225.
CrossRef - Edmundson M. C., Capeness M., Horsfall L. Exploring the potential of metallic nanoparticles within synthetic biology. New Biotechnology, 2014; 31(6): 572-578.
CrossRef - Ekor The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology, 2013;4: 177-186.
CrossRef - Elmowafy M., Viitala T., Ibrahim H. M., Abu-Elyazid S. K., Samy A., Kassem A., et al., Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur. J. Pharm. Sci., 2013;50:161-171.
CrossRef - Galib, Barve M., Mashru M., Jagtap C., Patgiri J., Prajapati P. K. Therapeutic potentials of metals in ancient India: A review through Charaka Samhita. Journal of Ayurveda and Integrative Medicine, 2011; 2(2): 55–63.
CrossRef - Geetha R., Ashokkumar T., Tamilselvan S., Govindaraju K., Sadiq M. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnology, 2013;4: 91-98.
CrossRef - Gomes A., Ghosh S., Sengupta J., Datta P., Gomes A. Herbonanoceuticals: A New Step towards Herbal Therapeutics. Medicinal and Aromatic Plants, 2014;3(3): 162. doi:10.4172/2167-0412.1000162.
CrossRef - Goyal A., Kumar S., Nagpal M., Singh I., Arora S. Potential of novel drug delivery systems for herbal drugs. Indian Journal of Pharmaceutical Education and Research, 2011; 45: 225-235.Greenwell, Rahman P. K. S. M. Medicinal Plants: Their Use in Anticancer Treatment. International Journal of Pharmaceutical Sciences and Research, 2015; 6(10): 4103–4112.
- Griggs B. Green Pharmacy: A History of Herbal Medicine. Viking Press, New York, 1982.
- Gunasekaran T., Haile T., Nigusse T., Dhanaraju M. D. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pacific Journal of Tropical Biomedicine, 2014;4(1): S1–S7.
CrossRef - Iram F., Iqbal M. S., Athar M. M., Saeed M. Z., Yasmeen A. Glucoxylan-mediated green synthesis of gold and silver nanoparticles and their phyto-toxicity study. Carbohydrate Polymers, 2014;104: 29-33.
CrossRef - Iravani S. Green synthesis of metal nanoparticles using plants. Green Chemistry, 2011;13: 2638-2650.
CrossRef - Jadhav N., Powar T., Shinde S., Nadaf S. Herbal nanoparticles: A patent review. Asian journal of Pharmaceutics, 2014; 8(1): 1-12.
CrossRef - Jantarat C. Bioavailability Enhancement Techniques of Herbal Medicine: A Case Example of Curcumin. International Journal of Pharmacy and Pharmaceutical Sciences, 2013;5(L): 493-500.
- Juzenas P., Chen W., Sun Y. P., Coelho M. A. N., Generalov R., Generalova N., Christensen I. L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Advanced Drug Delivery Reviews, 2008; 60: 1600-1614.
CrossRef - Kalele S., Gosavi S. W., Urban J., Kulkarni S. K. Nanoshell particles: synthesis, properties and applications. Current Science, 2006; 91(8):1038-1052.
- Kingston D. G. I. Modern natural products drug discovery and its relevance to biodiversity conservation. Journal of Natural Products, 2011; 74:496–511.
CrossRef - Krug H. F., Wick P. Nanotoxicology: an interdisciplinary challenge. Angewandte Chemie International Edition, 2011; 50(6): 1260-1278.
CrossRef - Kumar P., Kulkarni P. K., Srivastava A. A. Pharmaceutical application of nanoparticles in drug delivery system. Journal of Chemical and Pharmaceutical Research, 2015; 7(8): 703-712.
- Lin A., Li H., Liu Y., QIU X. Preparation and release characteristics of berberine chitosan nanoparticles in vitro. China Pharmacy, 2007;18: 755-757.
- Mahima, Rahal A., Deb R., Latheef S. K., Samad H. A., et al., Immunomodulatory and therapeutic potentials of herbal, traditional/indigenous and ethnoveterinary medicines. Pakistan Journal of Biological Sciences, 2012;15: 754-774.
CrossRef - Makarov V. V., Love A. J., Sinitsyna O. V., Makarova S. S., Yaminsky I. V., Taliansky M. E., Kalinina N. O. Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae, 2014; 6: 35–44.
CrossRef - Mamillapalli V., Atmakuri A. M., Khantamneni P. Nanoparticles for herbal extracts. Asian Journal of Pharmaceutics, 2016; 10(2): S54-S60.
- Manach C., Williamson G., Morand C., Scalbert A., Remesy C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies American Journal of Clinical Nutrition, 2005; 81: 230-242.
CrossRef - Mathur M. Achievements, constraints and gaps of nano-techniques pertains to augmenting herbal drug efficacy. Medicinal Plants, 2016; 8 (3): 171-198.
CrossRef - McNamara K., Tofail S. A. Nanosystems: the use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys Chem Chem Phys., 2015;17:27981–95.
CrossRef - Mukundan D., Mohankumar R., Vasanthakumari R. Green synthesis of silver nanoparticles using leaves extract of Bauhinia tomentosa Linn and its in vitro anticancer potential. Materials Today: Proceedings29 Part A, 2015; 4309-4316.
CrossRef - Nagavarma B. V. N., Yadav H. K. S., Ayaz A., Vasudha L. S., Shivakumar H. G. Different techniques for preparation of polymeric nanoparticles- a review Asian Journalof Pharmaceutical and Clinical Research, 2012; 5(3): 16-23.
- Naghsh F. Nano Drug Delivery Study of Anticancer Properties on Ginger using QM/MM Methods. Oriental Journal of Chemistry, 2015; 31(1): 465-478.
CrossRef - Pal, Sahu C. K., Haldar A. Bhasma: The ancient Indian nanomedicine. Journal of Advanced Pharmaceutical Technology & Research, 2014; 5(1): 4–12.
CrossRef - Papagiannaros A., Upponi J., Hartner W., Mongayt D., Levchenko T., Torchilin V. Quantum dot loaded immunomicelles for tumor imaging. BMC Medical Imaging, 2010;10(1): 22.
CrossRef - Park K. Controlled drug delivery systems: Past forward and future back. Journal of Control Release, 2014; 190: 3-8.
CrossRef - Patwardhan B., Vaidya A. D., Chorghade M. Ayurveda and natural products drug discovery. Current Science-Bangalore, 2004; 86: 789-799.
- Paul S., Chugh A. Assessing the role of Ayurvedic ‘Bhasmas’ as Ethno-nanomedicine in the metal based nanomedicine patent regime. Journal of Intellectual Disability Research, 2011;16: 509-515.
- Pavani T., Chakra Ch. S., Rao K. V. A green approach for the synthesis of nano-sized iron oxide, by Indian Ayurvedic modified bhasmikaran method. American Journal of Pharmaceutical Sciences, 2013;1: 1-7.
- Priprem A., Sutthiparinyanont S., Young J. S., Chulasiri M. Effect of formulations of nanosized quercetin liposomes on COX-2 and NF-kB in MCF-10A cells. Pharm. Nanotechnol., 2015; 1:26-34.
CrossRef - Rao P. V., Nallappan D., Madhavi K., Rahman S, Wei J.,Gan S. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxidative Medicine and Cellular Longevity, 2016; Article ID 3685671, 15 pages http://dx.doi.org/10.1155/2016/3685671.
CrossRef - Sachan A. K., Gupta A. A review on nanotized herbal drugs. International Journal of Pharmaceutical Sciences and Research, 2015; 6(3): 961-970.
- Sadat S. M. A., Jahan S. T., Haddadi A. Effects of Size and Surface Charge of Polymeric Nanoparticles on in vitro and in vivo Applications. Journal of Biomaterials and Nanobiotechnology, 2016; 7: 91-108.
CrossRef - Sarkar P. K., Das S., Prajapati P. K. Ancient concept of metal pharmacology based on Ayurvedic literature. Ancient Science of Life, 2010;29: 1-6.
- Shah M., Fawcett D., Sharma S., Tripathy S. K. , Poinern G. E. J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials, 2015; 8: 7278–7308.
CrossRef - Sharma A., Mitkare S., Moon R. Multicomponent herbal therapy: A review. International Journal of Pharmaceutical Sciences Review and Research, 2011; 6: 185-187.
- Sharma C., Singh C. Nano Carriers of Novel Drug Delivery System for “Ayurveda Herbal Remedies” Need of Hour– A Bird’s Eye View. American Journal of PharmTech Research 2014; 4(2): 60-69.
- Singhana B., Slattery P., Chen A., Wallace M., Melancon M. P. Light-activatable gold nanoshells for drug delivery applications. AAPS PharmSciTech, 2014; 15(3): 741-752.
CrossRef - Tan B. J., Liu Y., Chang K. L., Lim B. K., Chiu G. N. Perorally Active Nanomicellar Formulation of Quercetin in the Treatment of Lung Cancer. International Journal of Nanomedicine, 2012;7: 651-661.
CrossRef - Tan W. B., Zhang Y. Surface modification of gold and quantum dot nanoparticles with chitosan for bioapplications. Journal of Biomedical Materials Research Part A, 2005; 75: 56-62.
CrossRef - Thillaivanan S., Samraj K. Challenges, Constraints and Opportunities in Herbal Medicines – A Review. International Journal of Herbal Medicine, 2014; 2 (1): 21-24.
- Tsoi K. M., Dai Q., Alman B. A., Chan W. C. W. Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Accounts of Chemical Research, 2012; 46(3): 662-671.
CrossRef - Voigt ,Henrich-Noack P., Kockentiedt S., Hintz W., Tomas J., Sabel B. A. Toxicity of polymeric nanoparticles in vivo and in vitro. Journal of Nanoparticle Research, 2014; 16(6): 2379 https://doi.org/10.1007/s11051-014-2379-1.
CrossRef - Wang X., Podila R., Shannahan J. H., Rao A. M., Brown M. Intravenously delivered graphenenanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. International Journal of Nanomedicine, 2013; 8: 1733–1748.
CrossRef - Yadav D., Suri S., Choudhary A. A., Sikender M., Hemant Beg N. M. Novel approach: Herbal remedies and natural products in pharmaceutical science as nano drug delivery systems. International Journal of PharmTechResearch,2011; 3: 3092–3116.
- Yadav M., Bhatia V. J., Doshi G., Shastri K. Novel techniques in herbal drug delivery systems. International Journal of Pharmaceutical Sciences Review and Research, 2014; 28(2):83–89.
- Yildirimer L., Thanh N. T. K., Loizidou M., Seifalian A. M. Toxicology and clinical potential of nanoparticles. Nanotoday, 2011; 6(6): 585–607.
CrossRef - Yue P. F., Yuan H. L., Li X. Y., Yang M., Zhu W. F. Process optimization, characterization and evaluation in vivo of oxymatrine–phospholipid complex. International Journal of Pharmaceutics, 2010; 387: 139-146.
CrossRef - Zabolotnyi M. A., Momot A. I., Dovbeshko G. I., Gnatyuk E. P., Solyanyk G. I., Dmytrenko O. P., Kulish N. P., Fedina K. V. Modification of Alkaloid Structure in The Conium Drug With Fullerenes C60 M.A. Ukrainian Journal of Physics, 2012; 57(7): 739-745.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





