Manuscript accepted on : 11-April-2019
Published online on: 23-05-2019
Plagiarism Check: Yes
Reviewed by: Priyanka Singh
Second Review by: Varucha Misra
Impact of Plant Growth Regulators on Greek oregano Micropropagation and Antioxidant Activity
Ely Georgieva Zayova, Maria Prokopova Geneva* , Kamelia Dimitrova Miladinova-Georgieva, Marieta Georgieva Hristozkova and Ira Valkova Stancheva
, Kamelia Dimitrova Miladinova-Georgieva, Marieta Georgieva Hristozkova and Ira Valkova Stancheva
Institute of Plant Physiology and Genetics, Bulgarian Academy of Sciences, Acad. G. Bonchev Street, Bldg. 21, 1113 Sofia, Bulgaria.
Corresponding Author E-mail: boykova2@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2746
ABSTRACT: This study highlights the development and achievements made for the micropropagation of Greek oregano (Origanum heracleoticum L. ) using stem tip explants. The shoots were cultured on Murashige and Skoog (MS) medium followed different concentrations of plant growth regulators (PGR) - 6-benzyl aminopurine, thidiazuron and zeatin at concentrations (0.5 or 1.0 mg L-1). The induction of multiple shoots from stem tip segments was the highest in MS medium supplemented with 1.0 mg L-1 zeatin. It was the most effective medium for shoot formation, which produced multiple shoots (2.7) with an average height of 3.5 cm. These shoots were transferred on half strength MS medium containing three different auxins: indole-3-butyric acid, α-naphthalene acetic acid or indole-3-acetic acid (0, 0.1 and 0.5 mg L-1) for rooting, Multiple shoots were the most efficiently rooted on ½ MS medium supplemented with 0.5 mg L-1indole-3-butyric acid. Rooted plants showed the best adaptation on pots containing peat: perlite (2: 1 v/v). The higher rates of shoots number and height per plant have a positive relationship with the production of metabolites with antioxidant potential as phenols and flavonoids as well as with ferric reducing antioxidant potential.
KEYWORDS: Antioxidant Activity; Ex Vitro Acclimatization; Micropropagation; Origanum Heracleoticum
Download this article as:| Copy the following to cite this article: Zayova E. G, Geneva M. P, Miladinova-Georgieva K. D, Hristozkova M. G, Stancheva I. V. Impact of Plant Growth Regulators on Greek oregano Micropropagation and Antioxidant Activity. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Zayova E. G, Geneva M. P, Miladinova-Georgieva K. D, Hristozkova M. G, Stancheva I. V. Impact of Plant Growth Regulators on Greek oregano Micropropagation and Antioxidant Activity. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2VYQlja |
Introduction
Greek oregano (Origanum heracleoticum L.) is a perennial herb, naturally spread from the Mediterranean to Central Asia. Greek oregano has long been recognized as a culinary herb and medicinal plant (MP) in the food and pharmaceutical industry1. The essential oils from this plant have strong antioxidant and antibacterial activities2. They are insulated from 48 to 64 compounds constituting the oil as the main ingredients are carvacrol and thymol, followed by p-cement and γ-terpinene3, 4.
In contrast to conventional methods of propagation, vegetative micropropagation, using the tip and nodal segments, allows the production of a largely homogeneous population of plants in a relatively short period of time, irrespective of the season5. However, attempts to increase the amount of highly valuable metabolites by applying biotechnological methods for the propagation of O. heracleoticum appear to be rather limited. Full strength Gamborg’s B5 medium with 20 mg L-1 sucrose, 6 g L-1 agar supplemented with factorial combinations of 6-benzylaminopurine (BAP) (0.25 and 0.50 mg L-1), and 0.01 mg L-1 α-naphthalene acetic acid (NAA) has been used for Greek oregano micropropagation. For in vitro rooting, optimal development of shoots achieved by adding 0.5 mg L-1 NAA6. Morone-Fortunato and Avato7 reported that the best results for micropropagation in terms of percentage of growth, a number of developing shoots and shoot length of Greek oregano have been adding 0.5 mg L-1 BAP on a nutrient MS medium. Rooting of Green oregano was realized in a nutrient medium containing 0.15 mg L-1 of IAA8. Goleniowski et al.9 developed a protocol for in vitro propagation of Origanum vulgare × applici, by studying the effects of the BA and NAA at various concentrations and combinations on micropropagation.
The Greek oregano could reproduce by seeds germination and in vitro micropropagation. The small seeds have a good germination rate10. Unfortunately, this way of propagation has a very considerable disadvantage – as a cross-pollinating plant the offspring of seeds will represent a various population in relation to habitus, colouration, content and odour of the essential oil. Using plant micropropagation, allows the preservation of the genotypic and phenotypic characteristics of the initial plants as well plantlets are free from seasonal and somatic variations, infestations of bacteria, fungi, or insects and environmental pollution that can affect their medicinal value. Faisal et al.11 found that the in vitro propagated plants Bacopa monnieri L. using TDZ were genetically uniform to their mother plants. On the other hand, the choice of cytokinin type and concentration exogenously supplied to the nutrient medium during plant shooting markedly influences not only shoot proliferation but also the production of metabolites with antioxidant activity12. There is a lack of data in the scientific papers about changes of antioxidant potential of extracts in plantlets from in vitro propagated O. heracleoticum depending on the type and concentration of PGRs added to the nutrient medium for plant micropropagation.
The main purpose of this study was the influences of three cytokinins on shoot initiation and multiplication rooting and antioxidant activity of O. heracleoticum. This method will allow the reproduction of an unlimited number of plants with strictly specified qualities.
Material and Methods
The plant material used for the experiment in this study was a mature 2-year-old plant of O. vulgare L. ssp. hirtum (Link) Ietswaart (O. heracleoticum L.) collected from the greenhouse, Institute of Plant Physiology and Genetics, Bulgarian Academy of Sciences. Stem tips about 0.8-1.0 cm were used as a source of explants which were washed in running tap water and surface sterilized with 0.04% HgCl2 for 20 min followed by rinsing them three times with sterile distilled water for 15 minutes against fungal and bacterial spores.
The explants (stem tip segment) were cut with a sterile blade and inoculated on MS13 medium with 500 mg L-1 CaCl2, 30 mg L-1 sucrose, 7 g L-1 agar for two passages (passage – 3 weeks). The plantlets were prepared aseptically and were implanted on MS medium prepared with different concentrations of PGRs: 6-benzylaminopurine (BAP), thidiazuron (TDZ) and zeatin (0.5 and 1.0 mg L-1) for shoot proliferation and multiplication. The efficacy of the cytokinin concentration was determined by recording the percentage of formed shoots, the number of developing shoots per explant, shoot height and fresh weight and the length after three weeks of culture.
Rooting of elongated shoots (shoots obtained on MS + 1.0 mg L-1 zeatin) was attempted under in vitro conditions. Auxins (indole-3-butyric acid (IBA), α-naphthalene acetic acid (NAA) or indole-3-acetic acid (IAA) (0.1 and 0.5 mg L-1) were incorporated in the agar (0.7%) solidified medium containing ½ MS salts and 2.0% sucrose. As control medium was used ½ MS basal medium. Data were recorded on percentage rooting, the mean number of roots per plant, and root length after four weeks of culture.
The well-rooted plants were carefully taken out from the vessels and washed under running tap water to remove the gelling agent for ex vitro adaptation. The plants (shoots obtained on MS + 1.0 mg L-1 zeatin and rooted on MS + 0.5 mg L-1 IBA) were transplanted in each potting mixture. The in vitro rooted plants were transferred to 10 cm diameter pots filled with M1 soil: sand: perlite (2: 1: 1 v/v/v) and M2 peat: perlite (2: 1 v/v) for two months. Ex vitro acclimatization of plants was carried out at 24±2°C and 70-80% relative humidity under 16 h illuminations (50 μmol m-2s-1). There were ten plants per treatment. The pots were covered with porous polyethylene bags for maintaining high humidity (90%). The polyethylene was removed after two weeks. The per cent of survived plants, plant height and a number of leaves were measured after eight weeks.
For the antioxidant testing dry samples (0.3 g) from three weeks in vitro shooted plantlets were ground and extracted with 96% (v/v) methanol. Free radical-scavenging activity by using coloured, artificial stable free radicals DPPH• (1,1-diphenyl-2-picrylhydrazyl), was determined spectrophotometrically14. The percent inhibition of the DPPH• radical (I %) was calculated by the following equation: I% = [(Ablank – Asample)/Ablank] × 100, where, Ablank is the absorbance of the control reaction (containing all reagents except the extract), and Asample is the absorbance of the extract. The ferric reducing antioxidant power (FRAP) was monitored by BENZIE and STRAIN15. Concentrations of total phenolic compounds were determined spectrophotometrically using the Folin–Ciocalteu reagent and calculated as caffeic acid equivalents16. Flavonoids in plant tissues were measured spectrophotometrically according to Zhishen et al.17, using the standard curve of catechin.
The extraction for the determination of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and guaiacol peroxidase (GPO) activities was made by Hristozkova et al.18. Total SOD (EC 1.15.1.1) activity was determined by Giannopolitis and RIES19. Total CAT (EC 1.11.1.6) activity was measured according to Beers and Sizer20. Total APX (EC 1.11.1.1) activity was assayed according to Nakano and Asada21. Total GPO (EC 1.11.1.7) activity was determined by Urbanek et al.22. Soluble protein content was determined by Bradford23 using bovine serum albumin as a standard.
Statistical Analysis
The data were statistically processed by analysis of variance (ANOVA) for comparison of means, and significant differences were calculated according to Fisher’s least significance difference (LSD) test at the 5% significance level using a statistical software package (Statgraphics Plus, version 5.1 for Windows).
Results and Discussion
The growing conditions influenced favourably the plant development, without traits of deviation of their normal development, such as glassing, chlorosis, necrosis etc in cultivation on MS medium (Fig. 1a). Adding on MS medium BAP, TDZ or Zeatin (0.5 and 1.0 mg L-1) for Greek oregano shooting the maximum frequency (80%) was observed on MS medium supplemented with 1.0 mg L-1 zeatin, which induced a number of shoots (3.8) with a shoot height (3.9 cm) after three weeks of culture (Table 1, Fig. 1b). The concentration of 0.5 mg L-1 zeatin gave the explant responses in terms of percentage of shoot formation (70%), number of developing shoots (3.4), shoot length (3.6 cm), follow 1.0 mg L-1 BAP gave the number of developing shoots (2.7) and shoot length (3.5 cm) (Fig. 1c). The highest fresh weight values (0.19 g) were on MS medium with 1.0 mg L-1 zeatin, followed by fresh weight (0.16 g) on MS medium with 0.5 mg L-1 zeatin. Accordingly, the most of the published protocols for in vitro multiplication of MPs the type and concentration of the cytokinin significantly influence the frequency of the formed buds and buds average number of explant24,25. The protocols allowed the establishment of in vitro tip explants and also permitted in vitro multiplication of an adult plant with desirable agronomic features. Established conditions for maintaining growth and plant development cannot be applied with guaranteed success to all the representatives of a particular species, as the process is genotypically dependent. Protocols for in vitro multiplication of O. heracleoticum have been developed6,7. The investigation of multiplication protocol for O. heracleoticum was based on the independent addition of cytokinins (BAP, TDZ or Zeatin) to the MS medium. Greek oregano had a high in vitro multiplication factor in MS medium supplemented with 1.0 mg L-1 zeatin and gave the best responses in terms of percentage of growth, number of developing shoots and shoot height.
 |
Figure 1: Micropropagation of O. heracleoticum: a) In vitro shoots with 500 mg L-1 CaCl2; b) in vitro propagated shoots with 1.0 mg L-1 zeatin after three weeks of culture; c) in vitro propagated shoots with 1.0 mg L-1 BAP after three weeks of culture; d) rooted plants with 0.5 mg L-1 IBA after 4 weeks of culture; e) rooted plants with 0.5 mg L-1 NAA after 4 weeks of culture, f) ex vitro adapted plants after eight weeks of culture.
|
Table 1: Effect of plant growth regulators (PGRs) on shoot multiplication of O. heracleoticum shoots after 3 weeks of culture.
| PGRs, mg L-1 | Shoot formation % | Number of shoots explant-1 | Shoot height, cm | Fresh weight g |
| BAP | ||||
| 0.5 | 50 | 2.0±0.1a | 2.2±0.11a | 0.07±0.004a |
| 1.0 | 70 | 2.7±0.135bc | 3.5±0.175c | 0.13±0.006b |
| Zeatin | ||||
| 0.5 | 70 | 3.4±0.17d | 3.6±0.18c | 0.16±0.008c |
| 1.0 | 80 | 3.8±0.19e | 3.9±0.195d | 0.19±0.01d |
| TDZ | ||||
| 0.5 | 60 | 2.5±0.125b | 2.7±0.135b | 0.15±0.007c |
| 1.0 | 65 | 2.8±0.14c | 2.4±0.12a | 0.13±0.006b |
| LSD | 0.2603 | 0.2773 | 0.0126 |
The data are presented as means of 10 plants per treatment ± standard error. Different letters indicate significant differences assessed by Fisher LSD test (5%) after performing ANOVA multi-factor analysis.
The efficacy of IBA, NAA and IAA supplemented to the ½ MS media on rooting of O. heracleoticum plantlets is shown in Table 2. With increasing concentration from 0.1 mg L-1 to 0.5 mg L-1 IBA the healthy rooting percentage reached to 80% on ½ MS induced maximum average number (3.3) and length of roots (2.4 cm) after three weeks of culture (Table 2, Fig. 1d). The rooting response was observed on ½ MS medium containing 0.5 mg L-1 NAA, where was formed a number of roots per plant 2.8 with 1.5 cm root length (Table 2, Fig. 1e). Typical for the vegetative part of the plants was the good growing, the normal development, and the intensive green colour of the leaves. The roots were white, firmly fixed to the base of the plants, which was without callus. In most cases 0.1-0.5 mg L-1 IAA there were single ones, and the average percentage of rooting was lowest (40-50%). In the ½ MS medium with 0.1-0.5 mg L-1 IAA provokes some negative phenomena. The plants are growing but there are already pale, yellowish, and the lower leaves of most of them were fully necrotized at the end of the passage. Single roots were observed on the medium supplemented with 0.5 mg/L IAA (Table 2). The percentage of rooting (50%) was recorded. The rooting was 0% on control ½ MS medium. However, IBA and NAA were found to be the best rooting hormone than IAA in the ½ MS medium. The rooting medium for plants cloning often is modified by the inclusion of auxin. Nanova and Slavova8 reported that optimal rooting effect (89.6%) is reached under influence of 0.15 mg/L IAA in MS medium. Rooting of the 15–20 mm long shoots of Greek oregano was achieved in ½ B5 medium supplemented with 1% sucrose and either 10–6 M IBA or NAA (Kintzios 2002). In our previous studies has been recommending for in vitro Thymus vulgaris rhizogenesis inclusion of auxins and successful adaptation under ex vitro conditions25.
Table 2: Effect of ½ MS medium with auxins on the root induction of O. heracleoticum plants after 4 weeks of culture.
| Auxin, mg L-1 | Rooting % | Number of roots plant-1 | Root length cm |
| Control, ½ MS | 0 | 0 | 0 |
| ½ MS + 0.1 IBA
½ MS + 0.5 IBA |
45
80 |
1.7±0.085c
3.3±0.165f |
1.2±0.06b
2.4±0.12e |
| ½ MS + 0.1 NAA
½ MS + 0.5 NAA |
30
65 |
1.4±0.07b
2.8±0.14e |
0.9±0.045a
1.5±0.075c |
| ½ MS + 0.1 IAA
½ MS + 0.5 IAA LSD |
40
50 |
1.2±0.06a
2.2±0.11d 0.1984 |
0.8±0.04a
1.7±0.085d 0.1349 |
The data are presented as means of 10 plants per treatment ± standard error. Different letters indicate significant differences assessed by Fisher LSD test (5%) after performing ANOVA multi-factor analysis.
The transferring of rooted Greek oregano plantlets at ex vitro conditions was realized after three mounts of cultivation, in a mixture: Mix1 – soil, sand and perlite (2: 1: 1 v/v/v) and Mix 2 – peat: perlite (2: 1 v/v) (Table 3). The highest percentage (80%) of plantlets survival was recorded in a mixture of peat: perlite with plant height (6.1 cm) as well as the great number of leaves (5.3) on mixture Mix 2 and acclimatized plants appeared normal (Fig. 1f). They did not show any morphological abnormalities or variations. It was found that 60% of adapted plants survived after transplanting on Mix 1 with the plant height (4.2 cm) and a number of leaves (4.5). Usually, after one-month cultivation, their vegetative part was trimmed next to the lowest level, situated above the soil surface. After this manipulation was stimulated the development of more than one vegetative part from the grounds of the plants and until the end of the second month they were ready for planting in greenhouse conditions. A number of authors report successful adaptation of micropropaged plants under ex vitro conditions25, 26, 27. Micropropagated plants tend to acclimatize rapidly (three weeks) in the greenhouse at a high rate (95%) and usually are more vigorous than seed-propagated plants27. The soil substrate was successfully selected (1: 4 – humustim: a mixture of 1: 1 sand and pearlite) for rooting of Greek oregano plants after their one-month ex vitro adaptation8.
Table 3: Effect of mixture substrate on the survival of O. heracleoticum plants after 6 weeks of culture.
| Mixture | Plant survival % | Plant height cm | Number of leaves plant-1 |
| M1 – soil: sand: perlite (2: 1: 1, v/v/v) | 60 | 4.2±0.21a | 4.5±0.225a |
| M2 – peat: perlite (2: 1, v/v/)
LSD |
80 | 6.1±0.305b
0.5936 |
5.3±0.265b
0.5573 |
The data are presented as means of 10 plants per treatment ± standard error. Different letters indicate significant differences assessed by Fisher LSD test (5%) after performing ANOVA multi-factor analysis.
The enzyme antioxidant potential, estimated as SOD, CAT, GPO and APX activity was greatly influenced by PGRs BAP, Zeatin and TDZ used for O. heracleoticum micropropagation. When plants were in vitro propagated with higher concentration BAP (1.0 mg L-1), the enzymes activities decreased, while when have been used zeatin and TDZ the activity increased (Fig. 2). The same models of changes of the content of total phenols and flavonoids as well as the antioxidant activities of the Greek oregano plantlets extract assayed by free radical-scavenging activity (DPPH) and ferric reducing antioxidant power (FRAP) were recorded (Fig. 3).
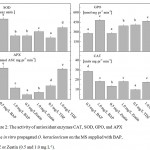 |
Figure 2: The activity of antioxidant enzymes CAT, SOD, GPO, and APX in the in vitro propagated O. heracleoticum on the MS supplied with BAP, TDZ or Zeatin (0.5 and 1.0 mg L-1).
|
Values are means ± SE, n=3; different letters indicate significant differences assessed by Fisher LSD test (P≤0.05) after performing ANOVA multi-factor analysis.
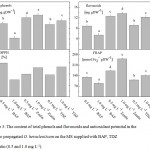 |
Figure 3: The content of total phenols and flavonoids and antioxidant potential in the in vitro propagated O. heracleoticum on the MS supplied with BAP, TDZ or Zeatin (0.5 and 1.0 mg L-1).
|
Values are means ± SE, n=3; letters in common within a graph indicate no significant differences assessed by Fisher LSD test (P≤0.05) after performing ANOVA.
Addition of PGRs as auxins and cytokinins on MS is necessary to induce shooting and rooting because they are key factors and strongly participate in cell cycle regulation and cell division28,29,30. PGRs influencing essential oil production31, but little is known about their effects on antioxidant enzymes and metabolites of in vitro cultures. The environment inside the tubes used in O. heracleoticum micropropagation is characterized by high relative humidity, the poor gaseous exchange between the internal atmosphere of the tube and its surrounding environment. Those conditions may induce physiological disorders. Plants protect themselves against the effect of the oxidative damage by antioxidant enzymes and low molecular secondary metabolites with antioxidant potential. The activities of enzymes SOD, CAT, GPO, and APX were increased with increased concentration of zeatin and TDZ, while it decreased when was used BAP. PGRs modify the plant growth and development pattern exerting a profound influence on many physiological processes32. Accordingly to SANTOS-GOMES et al.33 the total phenol content in sage (Salvia officinalis L.) leaf extract is changed depending on the type of cytokines adding to the nutrient medium during micropropagation. The authors reported that the lowest value of specific production of total antioxidant phenolic have occurred when plants are micropropagated with the supplementation of 1.5 mg L-1 benzyladenine. However, under these conditions, the shoot proliferation and linear shoot growth have been higher. In contrast, our data showed that the highest height and number of shoot per explant in variants with MS medium supplemented with 0.5 and 1.0 mg L-1 zeatin are in correlation with the highest content of total phenols and flavonoids. In general, high rates of biomass accumulation have a positive relationship with the production of secondary metabolites as phenols and flavonoids as well as with ferric reducing antioxidant potential.
Conclusion
The stem tip explants were frequently used for micropropagation of O. heracleoticum. The presence of 1.0 mg L-1 zeatin alone on MS medium was proved best for the multiple shoot formation of Greek oregano (average number of shoots 3.8, average shoot height 3.9 cm). The best rooting was observed on ½ MS medium containing 0.5 mg L-1 IBA, where a number of roots plant (3.3) measuring an average 2.4 cm were formed. The culture of O. heracleoticum has great potential for promotion and industrial production, one of the important MPs. Well-developed techniques are currently available to help growers meet the demand of the pharmaceutical and food industry. The higher antioxidant potential (determined by the content of total phenols and flavonoids and antioxidant enzymes activity) was recorded when MS medium was supplied with higher zeatin or TDZ concentration for O. heracleoticum shooting. On the contrary, using BAP as cytokine for shooting the antioxidant potential decreased.
Acknowledgements
This study was conducted with financial support from the National Science Fund at the Bulgarian Ministry of Education and Science, Project DN06/7 17.12.16.
Conflict of Interest
There is no conflict of interest.
References
- Baricevic D. and Bartol T. The biological/pharmacological activity of the Origanum genus. In: Medicinal and aromatic plants-Industrial profiles, Oregano. The genera Origanum and Lippia, S. E. Kintzios ed., vol. 25, CRC Press, London, 2002; 177-213.
- Karakaya S., El S., Karagözlü N. and Şahin, S. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgaressp. hirtum) by using different extraction methods. J. Med. Food, 2011; 14(6): 645-652.
CrossRef - Goliaris A.H., Chatzopoulou P.S. and Katsiotis S.T. Production of new Greek oregano clones and analysis of their essential oils. J Herbs Spec. Med. Plants, 2002; 10(1): 29-35.
CrossRef - Hussain A., Anwar F., Rasheed S., Nigam P., Janneh O. and Sarker S. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Braz. J. Pharmac., 2011; 21(6): 943-952.
CrossRef - Kintzios S. The biotechnology of oregano (Origanum sp. and Lippia sp.). In: Medicinal and aromatic plants-Industrial profiles, Oregano. The genera Origanum and Lippia, S. Kintzios, ed.., Taylor and Francis, London, 2002; 25: 237-242.
- Iconomou-Petrovich G. and Nianiou-Obeidat I. Micropropagation of Origanum vulgare subsp. hirtum (Mt. Taygetos). In: Progress in Botanical Research, Tsekos I., Moustakas M., eds., Springer, Berlin, 1998; 509-512.
CrossRef - Morone-Fortunato I. and Avato P., Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Plant Cell Tiss. Organ Cult., 2008; 93: 139-149.
CrossRef - Nanova Z. and Slavova Y. Mass vegetative propagation of winter marjoram (Origanum vulgare ssp. Hirtum (Link) Jetswaart). Bul. J. Agr. Sci., 2006; 12: 531-536.
- Goleniowski M. E., Flamarique C., Bima P. Micropropagation of oregano (Origanum vulgare×applii) from meristem tips. In Vitro Cell. Dev. Biol. Plant, 2003; 39(2): 125-128.
CrossRef - Yankulov Y. Winter marjoram, In: Basic Aromatic Plants. Zemizdat, Sofia, 2000; 122-141 (Bg).
- Faisal M., Alatar A. A., El-Sheikha M. A., Abdel-Salama E. M. and Qahtan A. A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi, Ind. Crops Prod., 2018; 118: 173-179.
CrossRef - Amoo S., Aremu A. and Staden V. In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tiss. Org. Cult., 2012; 111(3): 345-358.
CrossRef - Murashige T. and Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant., 1962; 15: 473-497.
CrossRef - Tepe B., Sokmen M., Akpulat H. and Sokmen A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem., 2006; 95: 200–204.
CrossRef - Benzie I. and Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem., 1996; 239: 70-76.
CrossRef - Pfeffer H., Dannel F. and Römheld V. Are there connection between phenol metabolism, ascorbate metabolism and membrane integrity in leaves of boron-deficient sunflower plants? Physiol. Plant., 1998; 104: 479-485.
CrossRef - Zhishen J., Mengcheng T. and Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem., 1999; 64: 555-559.
CrossRef - Hristozkova M., Geneva M., Stancheva I., Iliev I. and Azcon-Aguilar C. Symbiotic association between golden berry (Physalis peruviana) and arbuscular mycorrhizal fungi in heavy metal-contaminated soil. J. Plant Prot. Res., 2017; 57: 173-184.
CrossRef - Giannopolitis C. N. and Ries S. K., Superoxide dismutases I. Occurrence in higher plants. Plant Physiol., 1977; 59: 309-314.
CrossRef - Beers F. and Sizer I. F., A spectrophotometric method for measuring breakdown of hydrogen peroxide by catalase. J. Biol. Chem., 1952; 195: 133-140.
- Nakano Y. and Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol., 1987; 28: 131-140.
- Urbanek H., Kuzniak-Gebarowska E. and Herka K., Elicitation of defence responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plant., 1991; 13: 43-50.
- Bradford M. M. A rapid and sensitive method for the estimation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 1976; 72: 248-254.
CrossRef - Sidhu Y. In vitro micropropagation of medicinal plants by tissue culture, The Plym. Stud. Sci., 2010; 4(1): 432-449.
- Zayova E., Stancheva I., Geneva M., Hristozkova M., Dimitrova L., Petrova M., Sichanova M., Salamon I. and Mudroncekova S. Arbuscular mycorrhizal fungi enhance antioxidant capacity of in vitro propagated garden thyme (Thymus vulgaris L.), Symbiosis, 2017; 74(3): 177-187.
CrossRef - Zayova E., Nikolova M., Dimitrova L. and Petrova M. Comparative study of in vitro, ex vitro and in vivo propagated Salvia hispanica (Chia) plants: morphometric analysis and antioxidant activity, AgroLife Sci. J., 2016; 5(2): 166-173.
- Baricevic D. Experiences with oregano (Origanum spp.) in Slovenia. In: Promoting the conservation and use of underutilized and neglected crops – Oregano, S. PADULOSI, ed., 1997; 14: 111–121.
- Francis D. and Sorrell D. A. The interface between the cell cycle and plant growth regulators: a mini review. Plant Grow. Reg., 2001; 33: 1-12.
CrossRef - Fehér A., Pasternak T. P. and Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell,Tiss. Org. Cult., 2003; 74: 201-228.
CrossRef - Gaj M. D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Grow. Reg., 2004; 43: 27-47.
CrossRef - Prins C. L., Vieira I. J. C. and Freitas S. P. Growth regulators and essential oil production review, Braz. J. Plant Physiol. 2010; 22(2): 91-102.
CrossRef - Çavuşoğlu K., and Kabar K. The effects of pre-treatments of some plant growth regulators on germination and seedling growth of radish seeds under saline conditions. Dumlupinar Uni. J. Soc. Sci. 2007; 14: 27-35.
- Santos-Gomes P., Seabra R., Andrade P. and Fernandes-Ferreira M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.), Plant Sci., 2002; 162: 981-987.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





