Manuscript accepted on : 03-July-2019
Published online on: 30-06-2019
Plagiarism Check: Yes
Reviewed by: Ayush Dogra
Hassan I. H. El-Sayyad*1, Ahmed A. El-mansi2 and Samia M. Efekrin3
1Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt.
2Department of Biology, Faculty of Science, King Khalid University, Abha, KSA.
3Department of Zoology, Faculty of Science, Omar Al-Mukhtar University, Bayda, Libya.
Corresponding Author E-mail: elsayyad@mans.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2752
ABSTRACT: Increased consumption of processing food items rich in fat diet increased lipid laden products in body organs and developed obesity. It is also associated with the development of infertility. The present study designed to illustrate the developmental aspects of ovaries of offspring maternally fed on a high cholesterol diet and how supplementation of barley and date palm fruit to this die improved the ovarian structure and function. Ninety-six pregnant Wister albino rats categorized into eight groups (n=12); control (C), barley (B) (20%), dates (D) (20%) , barley & dates (10+10%) , hypercholesterolemic- (H), hypercholesterolemic & barley (H+B), hypercholesterolemic & dates (H+D) and hypercholesterolemic & barley & dates groups (H+B+D). Hypercholesterolemic diet (3% cholesterol) was intake for 6 weeks before conception and throughout gestation and lactation period. At 2 and 3 weeks post- partum, the offspring were sacrificed and their ovaries were removed and processed for histological, immunohistochemical and transmission electron microscopy. Sera and ovaries of the other groups were kept in refrigerator for biochemical investigations. The present findings revealed loss of ovarian follicles in offspring maternally fed in hypercholesterolemic groups associated with decreased expression of PCNA and over expression of caspase 3 and flow-cytometric analysis of annexin V manifesting cell death. At transmission electron microscopy, the ovaries of hypercholesterolemic group exhibited dense chromatin condensation of the nuclei of oocyte and follicle cells. On the other hand, the ovaries of offspring maternally fed on hypercholesterolemic diet plus barley and date palm fruit improved the decreased activities of catalase, superoxide dismutase and glutathione-s- transferase in comparison with the control. However, there was a marked increase of malondialdehyde, 8-hydroxy-2`-deoxyguanosine, caspase 3 and tumor necrosis factor-α in comparison with the control. Also, the sera levels of hyperocholesterolemic mothers such as follicle stimulating hormone, estradiol, and antimullerian hormone were almost retained to the normal level in animal groups fed on hypercholesterolemic diet containing dates /or barley grains. The authors finally concluded that offspring maternally fed on hypercholesterolemic diet developed reduction of ovarian follicular reserve, increases lipid peroxidation and altered maternal reproductive hormone involved in ovarian development. These dramatic alterations were improved post supplementing date palm fruits and/barley to the hypercholesterolemic diet.
KEYWORDS: Deoxyguanosine; Hypercholesterolemic; malondialdehyde
Download this article as:| Copy the following to cite this article: El-Sayyad H. I. H, El-mansi A. A, Efekrin S. M. Dietary Supplements of Barley and Date-Palm Fruit Improved the Growth Defects of Ovaries of Rat Offspring Maternally Fed on Hypercholesterolemic Diet. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: El-Sayyad H. I. H, El-mansi A. A, Efekrin S. M. Dietary Supplements of Barley and Date-Palm Fruit Improved the Growth Defects of Ovaries of Rat Offspring Maternally Fed on Hypercholesterolemic Diet. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2XY9bqB |
Introduction
Obesity and hypercholesterolemia are multifactorial in origin, and may result from overconsumption of a high cholesterol. They are associated with the development of atherosclerosis, hypertension, obesity, diabetes, and cancer (Ahmadizar et al., 2018; Baek et al., 2017; Ceska, 2017; Czubkowski et al., 2017). There is a close association between higher cholesterol diet and development of diabetes (El-Sayyad et al.,2010,2011,2014 ). High consumption of fat diets developed obesity leading to diabetes (Heydemann, 2016) and increase circulating glucose after 1 week of consumption (Winzell and Ahrén , 2004). Rats fed on a high fat diet for 15 weeks showed characteristic features of diabetes characterized by increased plasma level of TG, and glucose and decreased HDL-cholesterol (Ramalho et al., 2017).
Mice fed on a high fat diet increased the levels of pro-inflammatory cytokines, reduction in primordial follicles, and increased ovarian macrophage infiltration (Skaznik-Wikiel et al., 2016). In obese girls with central precocious puberty, increased BMI was associated with depleted LH levels at early pubertal stages (Lee et al., 2016). Ovaries from offspring maternally fed on a HF diet during weaning period showed a depletion of the small follicle numbers coincides with increased plasma estradiol levels and depletion of the luteinizing hormone at 6 months of age (Lin et al., 2017). Intake of high fat diet disorganised the hypothalamic-pituitary-adrenal axis through the increase of both leptin level and basal PRL receptor mRNA levels in the choroid plexus in both sexes of knockout mice (Kalyani et al., 2016). Obese women related to dysfunction of hypothalamic-pituitary-adrenal axis was found to increase of the serum cortisone level (Al-Safi et al., 2017) which impaired ovarian function (Rosmond and Björntorp, 2000).
On the other hand, fetuses maternally fed a HF diet developed failure of oocyte development (Léveillé et al., 2014; Tsoulis et al., 2016) as well as overexpression of genes associated cell death (FoXO3a), follicular growth (Gdf9) (Cheong et al., 2014). Also, the reduction of the antral and preovulatory follicles was closely related with the down-regulation of the gene expression involved in ovarian development as well as overexpression of genes related steroidogenesis synthesis such as oncreased kiss1 mRNA and kisspeptin protein (Zhou et al., 2019).
Wide traditional application of phyto-medicine paid a great attention for authors especially in decreasing the incidence of the development of metabolic diseases and supplied the body with the requirements of the antioxidant micronutrients. Barley is one of the most native cereal crops in middle east (Magnoliophyta, class Liliopsida (Monocotyledons), order: Oyperales, Family: Poaceae (Grass), Genus: Hordeum vulgare (Newman and Newman, 2008). It is rich in β-glucans which exert hypolepidemia especially of the cholesterol and triglyceride levels in animal model (Kalra and Jood, 2000) and human (McRorie and McKeown, 2017) via activates of the enzyme choleterol 7a-hydroxylase. This enzyme increases the transport of LDL cholesterol into the hepatocytes for conversion into bile acids thus derease the serum cholesterol and LDL cholesterol levels in the body (Abumweis et al., 2010; Wang et al., 2017). Also, it is rich in the antioxidants such as phenolic acids, flavonoids, lignans, tocols, phytosterols, and folate. These phytochemical components exhibited strong antioxidant, antiproliferative, and cholesterol lowering activities (Idehen et al., 2017) and cinnamic acid derivates, flavonoids, chalcones, tannins, quinones, proanthocyanidins and amino phenolic compounds (Dvorakova et al., 2008, Carvalho et al., 2015). On the other hand, date palm fruits are widely spread in middle east and rich in trace elements (Vayalil, 2012, Singh et al., 2012), vitamin A (β- carotene), B5, vitamins B1(thiamine) and B2 (riboflavin), vitamin B7 (niacin or nicotinic acid), vitamin C (ascorbic acid), biotin, pantothenic acid , B6, B9 and folic acid(folacin) (Baliga et al., 2011, Vayalil, 2012) and antioxidant micronutrients such as ferulic, gallic, catechin, chlorogenic, caffeic, coumaric, resorcinol, protocatechuic, dactyliferic, 3-o-caffeoylshikimic, sinapic, p-hydroxybenzoic, vanillic, syringic, procyanidin and isochlorogenic acids (Hamad et al., 2015).
Although the high fat diet and obesity were involved in reduction of ovarian follicles, however the mechanism of ovarian damage as well as the capacity of improvement post-supplementation of date palm fruits and barley are not illustrated and there is no available literature concerning it. This work aimed to illustrate the influence of a high cholesterol diet during intrauterine life and breast-feeding period on ovarian follicle differentiation via histo-cytological investigation, biochemical assessments of antioxidant capacity and maternal reproductive hormonal levels. At the same time illustrate how can barley and date-palm fruit diet plus high cholesterol diet can be improved the mentioned criteria of ovarian structure and function.
Materials and Method
Induction of Hypercholesterolemia
This was carried according to Enkhmaa et al.( 2005). The dietary components involve 3% cholesterol, 8% cocoa butter, 2% cholic acid and 1% thiouracil and the rest is standard diet formula plus minerals and vitamins. The hypercholesterolemic group maintained on the mentioned formula for 6 weeks before mating and through out gestation and lactation period at 1,2 & 3 weeks.
Diets Containing Barley And or Dates
The standard diet include either 20% barley or dates or 20% of both (10% dates plus 10% barley). Also, the hypercholesterolemic groups were fed on a diet containing either 20% barley and/ or date plam fruits according to the previously mentioned.
Experimental Work
One hundred and twenty eight fertile virgin female and males of albino rats weighing 100-110 g. body weight (at a ratio of 1 male: 3 females) were obtained from Hellwan Animal Breeding Farm (Ministry of Health, Cairo, EGYPT) and housed in plastic cages and maintained in a room at 23°C with a 12 h light- 12 h dark cycle. Free access of standard diet and water were allowed ad-libitum. Females were made pregnant by keeping them with healthy fertile males for 12 hour between 8 hour overnight till 8 hour in the next morning (at a ratio of 1 male -3 females). The pregnancy was determined by vaginal smear and detection of sperm to record the onset of gestation. The studied pregnant groups were arranged into eight groups (n=12); control (C), barley (B), dates (D), barley & dates, hypercholesterolemic- (H), hypercholesterolemic & barley (H+B), hypercholesterolemic & dates (H+D) and hypercholesterolemic & barley & dates groups (H+B+D).
At the end of treatment the offspring of the studied groups were sacrificed and their ovaries were removed and arranged for three parts, one for histological and immunohistochemical investigation and kept in 10% phosphate buffered formalin (pH 7.4), the second fixed in 2% glutaraldhyde in phosphate buffer (pH 7.4) for transmission electron microscopy. The mother of the studied were sacrificed and their blood was removed, centrifuged and serum was separated. Serum of mothers and ovaries of offspring were kept in refrigerator at -20°C for biochemical investigation.
Investigated Parameters
Histological and Morphometric Investigation
Ovary of breast-feeding offspring at 1.2 and three week-old were fixed immediately in 10% phosphate buffered formalin (pH 7.4), dehydrated in ascending series of ethyl alcohol, cleared in toluene and mounted in molten paraplast at 58-62°C. Five µm sections were cut and stained with Hematoxylin & eosin. For morphometric assessments, The number of oogonia, primary and secondary oocyte and teriary follicles were counted in five replicate slides from five specimens based on morphological classification (Li et al., 2013) using bright field Olympus light microscope (BX61 Olympus Optical Co. LTD, Japan).
Transmission Electron Microscopy (TEM)
Ovaries were fixed in 2.5 % cacodylate buffered glutaraldehyde buffered (pH 7.4) and post-fixed in 1 % osmium tetraoxide followed by dehydration in ascending grades of ethyl alcohol, cleared in propylene oxide and embedded in epoxy–resin. Ultrathin sections were cut on a LKB Ultratome IV (LKB Instruments, Bromma, Sweden) and mounted on grids, stained with uranyl acetate and lead citrate, and examined under a Joel 100CX transmission electron microscope (Musashino 3-chome, Akishima, Tokyo 196-8558, Japan).
Immunohistochemistry for Caspase-3 and PCNA
Five μm sections of formalin-fixed, ovarian tissues were kept on a polylysine-coated glass slides. After overnight packing at 65°C, the sections were deparaffinized and rehydrated in ethyl alcohol and placed in 0.05 % trypsin (pH 7.8) for 15 minutes at 37°C. These were followed by incubation with the primary antibody of caspase-3 (DAKO, clone MIB5, 1:50, mouse) and primary antibody against proliferating cell nuclear antigen (PCNA) at 1:50 overnight at 4°C. Then, the slides were incubated with a secondary biotin linked anti-mouse antibody and with the streptavidin-peroxidase complex. Sections were then incubated with the developing solution (diaminobenzidine-hydrogen peroxide; DAKO), and counterstained with hematoxylin. The immune reaction was visualized as brown reaction and counterstained with hematoxylin.
Biochemical Assays
At the end of treatment, offspring of both control and experimental groups were sacrificed and their ovaries were removed, homogenized in 10% ice-cold 2.5 mM-tris buffer (pH 7.5) and centrifuged at 14000 x g for 15 minutes at 4°C and the supernatant was kept in a refrigerator. Catalase activity was determined according to Bock et al. (1980). The reaction product was read at 240 nm. Superoxide dismutase (SOD) activity was determined according to Niskikimi et al. (1972) and its determination was based on the inhibition of nitroblue tetrazolium (NBT). by superoxide radicals to blue colored formazan and assayed at 560 nm. The glutathione-S-transferase activity is determined according to Habig et al. (1974) via catalyze the reaction of the -SH group of glutathione. Glutathione conjugates are metabolized into glutamate and glycine residues, followed by acetylation and formation the final product, a mercapturic acid and conjugated with 1- chloro- 2,4- dinitrobenzene and red the absorbance at 340 nm.
Lipid peroxidation end product malondialdhyde was determined according to Ohkawa et al. (1982). The method involved addition of 20µL of ovary homogenate supernatant to 100 μL of sodium dodecyl sulfate (SDS), 750 μL of 20% acetic acid (pH 3.5), 750 μL of 0.6% thiobarbituric acid, and 30 μL of distilled water and incubated at 95°C for 60 minutes. After cooling to the room temperature, 0.25 mL of butanol: pyridine (15:1) and 50 μL of distilled water were added, vortexed, and centrifuged at 2000rpm for 15 minutes. A reddish pink color was developed and estimated at 532 nm which indicates the extent of peroxidation and expressed as η mol/ mg protein. Standardization was carried out by using 1,1,3,3-tetraethoxypropane.
Determination of 8-hydroxy-2-deoxy guanosine was determined in ovaries by the Bioxytech 8-HdG -ELISA Kit (OXIS Health Products, Portland, OR, USA, Catalog. No. KOG-200S/E). Caspase-3 was determined by ELISA kit of a Stressgen kit (catalog No. 907-013). Tumor necrosis factor-α (TNF-α) was carried by using USCN Life Science Inc ELIZA Kit no. E99133Ra.
The serum level of antimullerian hormone, estradiol and follicle stimulating hormone were determined by using ELISA kit of Cusabio Biotech company.
Flow Cytometric Assessments of Cell Cycle M1 Apoptosis by Annexin
It was carried out by Becton Dickinson FACScan Fluorescence Activated Cell Analyzer (Becton Dickinson, Sunnyvale, CA, USA). The ovarian cells were lysed, suspended with Tris–EDTA buffer (pH 74) (Sigma-Aldrich Co.) and fixed in 80% ethanol. Cell suspension was prepared at a concentration of 0.1-0.3×106/ml and stained with fluorescein isothiocyanate-conjugated annexin V (annexin V-FITC) and assayed after incubation for 15 minutes at room temperature.
Statistical Analysis
Data were observed as a mean ± standard error (SE). The statistical analysis was performed with one-way post-hoc analysis of variance (ANOVA) using SPSS (version 13) software package for Windows, comparing the variations between studied groups and P<0.05 was considered statistically significant.
Results
Morpjometric Observations
From table (1), the ovaries of 3 week-old maternally fed on a hypercholesterolemic diet exhibited a marked decrease of the ovarian reserve of different stages of ovarian follicles in comparison with the control and or/ barley and date palm fruit supplementation. On the other hand, the ovaries of offspring maternally fed on a high cholesterol diet containing barley and/or date palm fruit restored the ovarian content of growing oocytes but were still not matched with the control.
Table 1: Total number and percentages of ovarian follicles in ovaries of 3 week-old offspring maternally fed on a hypercholesterolemic diets containing barley and/or date –palm fruits.
| Total number | Oogonia | Iry oocyte | 2ry oocyte | Tertiary ova | Atretic follicle | |
| Control | 216
(100%) |
104
(48.1%) |
48 (22.2%) | 36
(16.7%) |
28
(13%) |
– |
| Barley diet | 226 (104.6%) | 109
(48.2%) |
51
(22.6%) |
39
(37.3%) |
30
(13.3%) |
– |
| Dates diet | 194
(89.8%) |
108
(55.7%) |
32
(16.5%) |
32
(16.5%) |
22
(11.3%) |
– |
| Barley & dates diet | 179
(82.9%) |
112
(62.6%) |
42
(23.5%) |
28
(15.6%) |
26
(14.5%) |
– |
| Hypercholesterolemia | 156
(72.2%) |
78
(50%) |
34
(21.8%) |
29
(18.6%) |
18
(11.5%) |
24
(15.4%) |
| Hypercholesterolemia plus barley | 184
(85.2%) |
77
(41.8) |
56
(30.4%) |
35
(19.8%) |
12
(6.5%) |
4
(2.2%) |
| Hypercholesterolemia plus Dates | 172
(79.6%) |
74
(43.0%) |
37
(21.5%) |
30
(17.4%) |
23
(13.4%) |
8
(4.7%) |
| Hypercholesterolemia plus dates and barley | 175
(81%) |
71
(48.6%) |
38
(21.7%) |
32
(18.3%) |
28
(16%) |
6
(3.4%) |
Each result express the total number of ovarian follicles in five sections of 5 replicates at X250. The percentages expressed in relation to the control in case of total number. For each studied group. the total number represent 100% and every percentages of ovarian follicles are determined in relation to it.
Histo-& Cytological Observations of the Ovary
At 2 week-old, the ovary of the control is filled with normal oogonia, primary and secondary ovarian follicles separated from each other by a thin delicate collagenous fibrous sheath. The secondary oocytes varied from the primary ones by their marked increase of the overall follicular diameter as a result of multilayered covering follicle cells. Although the majority of the follicle cells remained cuboidal, the outermost layer in contact with the ovarian stroma become columnar in shape. A homogenous zona pellucida was only remarked in primary and early secondary oocyte. The oocyte possessed lightly stained eosinophilic cytoplasm and a nucleus, with a prominent darkly staining nucleolus. Numerous secondary-ovarian follicles are detected at different stages of growing antral cavity within the membrana granulosa (Fig. 1 A&B).
In ovaries of those of hypercholesterolemic mother, there was a marked increase of follicular atresia manifested by massive degeneration of oocytes with pyknotic nuclei and changes with the follicle cells. Some ovarian follicles possessed massive follicular atresia and their surrounding follicular cells become stuffed with fibrous sheath. Hyalinized degeneration of ovarian follicles was detected (Fig.1 C). Ovaries of offspring maternally fed on hypercholesterolemic containing barley exhibited marked improvement of the histological and cytological picture. These were characterized by normal structures of oogonia, primar and secondary oocytes. However, those of offspring maternally fed on hypercholesterolemic diet containing date or dates and barley showed improved structure but of less degree compared to ovaries of those fed on hypercholesterolemic containing barley and control (Fig.1D, E, F).
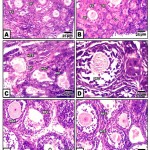 |
Figure 1: (A-F). Photomicrographs of histological sections of ovary of neonate 2 week-old.
|
At three weeks, the control ovary exhibited different generation of the growing oocytes including primary , secondary and multi-layered semi like mature ova. The mature oocytes are few in numbers and present in the cortico-medullary junction and possessed distinguished three layers in the follicular wall. The cumulus oophorus which enclosed the oocytes are composed of cuboidal granulosa cells. The outermost cell columnar cells are separated from the theca interna by a well-defined basement membrane . Both the membrane granulosa and the theca interna become progressively thinner during follicular development (Fig.2A&B). At ultrastructure level, the criteria of ovarian follicles with their outer covering follicle cells and normal pattern of nuclei and cytoplasm rich in mitochondria ( Fig.2 A-A2).
In ovaries of those of hypercholesterolemic mother, there were a marked increase of degenerated ovarian follicles (Fig.2 C). A marked degree of improvement histological picture was recorded in those fed on hypercholesterolemic diet containing barley comparing to those fed on hypercholesterolemic diet containing date or barley and date (Fig. 2 D,E,F).
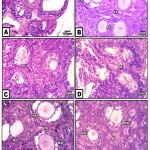 |
Figure 2: (A-F). Photomicrographs of histological sections of ovary of neonate 3 week-old.
|
A&B. Control & barley fed group showing stages of growing follicles. C. Maternally fed on hypercholesterolemic diet showing degeneration of growing follicles. D. Maternally fed on hypercholesterolemic diet containing date palm fruit showing less improvement of regenerated follicles. E. Maternally fed o n hypercholesterolemic diet containing barley and supplemented barley showing regenerated follicles. F. Maternally fed on hypercholesterolemic diet containing barley and dates showing a moderate improvement. Abbreviations; FC, follicle cell; DF, degenerated follicle cell; NSF, normal secondary follicle; NTE, normal theca externa; O, oogonia; Pr, primary oocyte; RFC, regenerated follicle cell; SF, secondary follicle; TE, theca externa.
At ultrastructural level, the ovarian follicles of offspring maternally fed on a high cholesterol diet exhibited massive degeneration of ovarian follicles characterized by pyknotic nuclei and degenerative phases of follicle cells. Karyolytic nuclei of degenerated oocytes were associated with increased accumulation of lysosomes in cytoplasm (Fig.3 B-B2).
On the other hand the ovarian follicles showed a marked increase of improvement in ovaries of those maternally fed on hypercholesterolemic diet containing barley, although dramatic alterations in some oocyte was still exist. The degree of improvement is moderate in those maternally fed on hypercholesterolemic diet containing either date or date and barley showed moderate improvement. The follicle cells restored almost their normal cytological pictures. Some oocyte exhibited pseudo-cleavage. The blood vessel exhibited normal endothelial lining cells (Fig.3 C-C2, D-D2).
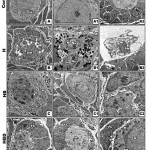 |
Figure 3: (A-D2). Transmission electron micrographs of ovary of 3week-old rat.
|
A-A3. Control showing ordinary structure of primary-(A1,A2) and secondary ovarian follicles. B-B2. Maternally fed on hypercholesterolemic diet showing damaged primary oocyte with vacuolated cytoplasm (B), pseudocleavage with damaged nuclei and abundant lysosomes in cytoplasm (B1) and damaged secondary follicle with pyknotic nuclei (B2). C-C2. Maternally fed on hypercholesterolemic diet containing barley showing improved ovarian follicles and their lining follicle cells. D-D2. Maternally fed on hypercholesterolemic diet containing barley showing improved ovarian follicles and their lining follicle cells. Abbreviations; D, desomosome; FC, follicle cell; M, Mitochondria; N, nuclei; NE, nuclear envelope; Nu, nucleoli. Star means zona pellucida.
Immunohistochemistry of PCNA and Caspase-3
In control, barley and date-supplemented groups, the ovarian tissues possessed overexpression of PCNA mostly in the proliferating follicle and stroma cells. In ovaries of neonates maternally fed on hypercholesterolemic diet, there was a marked reduction of the immunohistochemical reaction. However, in ovaries of those maternally fed on hypercholesterolemic diet containing barley and/or dates, there was a detected moderate dark-brown immunohistochemical reaction. The immunostaining reaction was highly observed in ovarian follicles of neonate maternally fed on hypercholesterolemic diet containing barley more than those of dates or barley and dates in comparison with the control (Fig. 4). Image analysis represented the average change of the expression of PCNA immunohistochemical reactions in the studied groups (Fig.5).
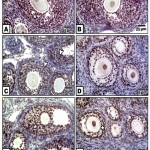 |
Figure 4: (A-F). Photomicrographs of formalin-fixed, paraffin-embedded ovary of 3 week old neonate immunohistochemically stained with the antibody of PCNA.
|
A & B. Control & barley showing iIncreased PCNA expression in follicle cells. C. Maternally fed on hypercholesterolemic diet showing decreased expression of PCNA in follicle cells. D. Maternally fed on hypercholesterolemic diet containing dates showing moderate expression of PCNA in follicle cells. .E. Hypercholesterolemic group supplemented barley showing increased expression of PCNA. F. Maternally fed on hypercholesterolemic diet containing barley and dates showing moderate expression of PCNA.* means overexpression of PCNA. Arrow head means decreased expression.
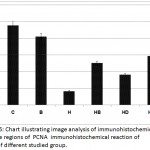 |
Figure 5: Chart illustrating image analysis of immunohistochemical reactive regions of PCNA immunohistochemical reaction of ovary of different studied group.
|
Concerning cysteine-aspartic acid protease 3 (caspase-3), the activity of caspase-3 was highly detected in the follicle and stromal cells of the ovaries of offspring maternally fed on a hypercholesterolemic diet compared to the control. On the other hand, the ovarian follicles of offspring maternally fed on a hypercholesterolemic diet containing barley and/ or dates possessed a decreased immunohistochemical reaction (Fig. 6). Image analysis revealed the varying intensity of the caspase-3 imunohistochemical reaction in the different experimental groups compared to the control. Marked reduction of the immunohistochemical reaction was detected in the ovarian follicles of offspring maternally fed on a hypercholesterolemic diet containing barley and/or dates (Fig. 7).
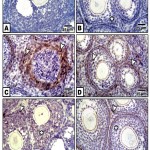 |
Figure 6: (A-F). Photomicrographs of formalin-fixed, paraffin-embedded ovary of 3 week old neonate immunohistochemically stained with the antibody of caspase 3.
|
A &B. Control & barley group showing a slight expression of caspase 3 in follicle and theca cells. C. Maternally fed on hypercholesterolemic diet showing overexpression of caspase 3 in follicle cells as expressed by arrow head. D. Maternally fed on hypercholesterolemic diet containing dates showing only expression of caspase in the theca cells. E. maternally fed on hypercholesterolemic diet containing barley showing decreased expression of caspase. F. Maternally fed on hypercholesterolemic diet containing barley and dates showing decreased expression of caspase which is limited to theca externa. Star mentioned decreased expression of caspase 3.
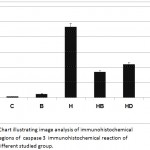 |
Figure 7: Chart illustrating image analysis of immunohistochemical reactive regions of caspase 3 immunohistochemical reaction of ovary of different studied group.
|
Biochemical Observations
Neonatal ovaries maternally fed on a hypercholesterolemic diet exhibited a marked decrease of the activities of catalase, superoxide dismutase and glutathione-s- transferase in comparison with the control. However, there was a marked increase of malondialdehyde, caspase 3, 8-hydroxy-2`-deoxyguanosine and tumor necrosis factor-α in comparison with the control. Meanwhile, the ovarian tissues of neonates maternally fed on the hypercholesterolemic diet containing barley and/or dates restored the assayed antioxidant levels and decreased the sharp rise of apoptic markers malondialdehyde, caspase 3and 8-hydroxy-2`-deoxyguanosine and decreased inflammation assessed by tumor necrosis factor-α but their levels were still not matched with the control (Table 2).
Table 2: Ovarian levels of antioxidants, malondialdehyde and apoptic markers of offspring maternally fed on a hypercholesterolemic diets containing barley and/or date –palm fruits.
| C | H | BH | DH | DBH | F-test | P≤ 0.05 | |
| CAT (U/10mg) | 11.6±0.6 | 9.9±0.4 | 10.8±0.5 | 10.9±0.4 | 11.17±0.6 | 0.67 | IS |
| GST(umol/10mg) | 18.4±0.6 | 12.9±0.5 | 14.0±1.4 | 13.1±0.5 | 14.5±0.6 | 21.11 | S |
| SOD (U/10mg) | 6.6±1.1 | 5.7±0.4 | 6.1±0.5 | 5.1±0.4 | 6.22±0.4 | 2.61 | IS |
| MDA (nmol/10mg | 1.9±0. 8 | 2.8±1. 8 | 2.4±0.8 | 2.5±1.7 | 2.23±1.3 | 0.60 | IS |
| 8HDG (pg10mg) | 3.9±0.3 | 6.7±0.44 | 5.1±0.5 | 3.1±0.7 | 5.32±0.6 | 1.47 | IS |
| CASP 3 (ng/ml) | 1.8±0.4 | 3.2±0.5 | 2.9±0.3 | 3.1±0.5 | 2.54±0.3 | 2.04 | IS |
| TNF (pg/10mg) | 28.2±10.4 | 328.5±0.6 | 316.8±6.4 | 332.7±7.47 | 300.2±8.4 | 261.64 | S |
Each result represents the mean ± SE (n=5), * Significant at P<0.05. Abbreviations; 8-hdg, 8- hydroxy guanosine; B, barley supplementation; B+D, barley and dates supplementation; C, control; caspase-3, cysteine-aspartic acid proteas 3; CAT, catalase; D, dates supplementation; Gst, glutathione S-transferase; H, hypercholesterolemia; H+B, hypercholesterolemia and barley; H+B+D, hypercholesterolemia, barley and dates; H+D, hypercholesterolemia and dates; MDA, malondialdehyde; SOD, superoxide dismutase enzyme; TNF, tumor necrosis factor.
From table (3), mothers fed on hypercholesterolemic diet exhibited marked increase of testosterone, estradiol, and antimullerian hormone and a decrease in follicle stimulating hormone. The abnormal change of the assayed hormonal levels were almost retained to the almost normal level in animal groups fed on hypercholesterolemic diet and /or date and barley grains.
Table 3: Serum hormonal level of mother rats fed on hypercholesterolemic diet and/or dates and barley.
| C | H | HB | HD | HBD | F-test | P ≤ 0.05 | |
| Estradiol (ng/ml) | 26.8±2.4 | 40. 6±3.6 | 35.4±3.6 | 37.4±0.6 | 34.4±0.6 | 69.36 | S |
| Follicle stimulating hormone (um/ml) | 43.06±2.2 | 38.71±2.7 | 50.03±10.8 | 38.65±0.51 | 40.78±0.48 | 0.90 | IS |
| Anti-Müllerian hormone (ng/ml) | 24.6±0.6 | 18.0±0.4 | 18.7±0.6 | 18.17±1.5 | 19.8±0.4 | 23.70 | S |
Each result represents the mean ± SE (n=5), * Significant at P<0.05. Abbreviations; 8-hdg, 8- hydroxy guanosine; B, barley supplementation; B+D, barley and dates supplementation; C, control; D, dates supplementation; H, hypercholesterolemia; H+B, hypercholesterolemia and barley; H+B+D, hypercholesterolemia, barley and dates; H+D, hypercholesterolemia and dates.
Flow Cytometry of Annexin V in Testis
From figure (8), the ovarian tissue of offspring maternally fed on a hypercholesterolemic diet exhibited a significant increase of the assayed annexin v of summation UR+LR manifesting apoptosis. On the other hand, those of mother fed on a hypercholesterolemic diet containing barley and/or dates exhibited a decrease of the assayed annexin v manifesting improvement especially in those of hypercholesterolemic and barley group.
 |
Figure 8: Flow cytometry chart of annexin V of ovary of offsprings maternally-fed on hypercholesterolemic diet and or dates and barley.
|
From annexin V chart: (1) UR showing positive annexin V and positive propidium iodide (PI), indicating late apoptosis. (2) UL showing positive annexin V and negative PI, indicating early apoptosis. (3) LL showing negative annexin V and negative propidium iodide (PI), indicating viable cells. (4) LR showing negative annexin V and positive propidium iodide (PI), indicating necrotic cells. UR plus LR, indicating the summation of apoptosis.
Discussion
Maternal high fat diet programmed the growth of obese offspring associated with inflammation and developmental of dysfunction in different body organs (Desai et al., 2014; Ingvorsen et al., 2015; Englich et al., 2017). The differentiation and proliferation of the ovarian follicles are more sensitive to intrauterine environment cytotoxicity and determined the developmental origin of the reproductive potential in adulthood, illustrate the prospective decline of the reproductive capacity later in life. The present work carried out during breast-feeding period 1, 2 and 3 weeks taking in consideration the generation of the primordial follicles started in late prenatal and early postnatal life.
From the present findings, maternal rat fed on hypercholesterolemic diet exhibited dramatic degeneration of primary and secondary ovarian follicle in 1-week old offspring and continuous in tertiary follicles in 2 and 3 week-old offspring. Follicular atresia associated with hyalinized antrum and surround follicle cells was markedly increased. The oocyte nuclei felled of grouping heterochromtin and abundant cytoplasmic vacuoles were detected. The follicle cells lacked normal characteristic structures and their nuclei appeared electron-dense.
The present data supported the work of Luzzo et al. (2012) and Zhou et al. (2019) in offspring of mice and rat maternally fed on a high fat diet. The studies mentioned only the loss of neonatal ovarian follicles. Similar findings of damaged ovarian follicles were also observed in obese girls (Lee et al., 2016).
Also, the damaged ovarian follicles were confirmed by the overexpression of caspase 3 immunohistochemical staining in follicle cells coincide with down-regulation of PCNA. The percentages of apoptic cells were markedly increased following flow-cytometric assessments of annexin v and increased ovarian level of ovarian 8-hydroxy-2`-deoxyguanosine and caspase 3.
Similar increase of apoptosis of granulosa cells were observed in mice fed on high fat diet (Wu et al., 2010; 2015).
Proliferating cell nuclear antigen (PCNA) ,is a key regulating proliferating cellular processes, and involved the exact information about the differentiation and developmental aspects of ovarian follicle development during neonatal life. Down-regulation of PCNA in cultured 18.5 day post conception mouse ovaries reduced the apoptosis of oocytes, accompanied by down-regulation of the pro-apoptotic genes; Bax, caspase-3, and TNFα and TNFR2 (Xu et al., 2011).
The observed increase cellularity in the ovarian stroma reflected inflammation and coincides with damage of the endothelial cells lining the blood capillaries and presence of abundant collagenous fibers in-between the atretic follicles. These findings were confirmed by increased level of tumor necrosis factor.
Maternal hypercholesterolemic was found to increase serum oxysterols level (Dumolt et al., 2018) which induce cell death in in vitro culture of bovine ovarian granulosa cells (Hall, 2006).
Kajihara et al. (2009) attributed dramatic loss of ovarian follicles and increase of follicular atresia in obese (fa/fa) Zucker rats to the overexpression of the nuclear accumulation of FOXO1 transcription factor in TUNEL-positive granulose cells.
Wu et al. (2015) attributed the damage in granulosa cells of ovaries of mice fed on a high fat diet to the cell cycle inhibitors, p27(Kip1) and p21(Cip1), and expression levels of Cyclin D1, D3 and CDK4.
Also, the breakdown of ovarian follicles in offspring of hypercholesterolemic mother reflected the increase of lipid peroxidation of ovarian malondialdhyde level decrease of the antioxidant defense of the assayed antioxidant enzymes superoxide dismutase and glutathione-s-transferase. It is known the antioxidant enzymes scavenge the free radicals liberated from the diseased tissue. If the free radicals exceed the antioxidant defense, it led to oxidation of cell biomolecules like DNA, protein and lipid materials leading to lipid peroxidation and dramatic damage of the tissues (Ighodaro and Akinloye, 2018). Catalase is involved in elimination of the reactive hydrogen peroxide species which alter the configuration of carbohydrates, nucleic acids, lipids, and proteins in living tissues. Superoxide dismutase (SOD) facilitated the change of superoxide is converted into hydrogen peroxide which can easily diffuses across the plasma membrane (Noor et al., 2002; McCord ,2008). Also, glutathione S-transferases (cytosolic GST, mitochondrial GST, 80, 81 and membrane-associated microsomal GST) are involved in removing the aldehyde products of lipid peroxidation via catalyzing its conjugation with glutathione (Tjalkens et al., 1998; Aiken et al., 2016).
Reduction of the assayed antioxidant enzymes catalase, SOD and glutathione-S-transferase in the ovarian cells facilitated the liberation of free radicals and increase of lipid peroxidation leading to damage of the ovarian cells specially follicle cells and oocytes.
Similar findings of decreased antioxidant enzymes were observed in mice (Aiken et al., 2016) and pig offspring (Xu et al., 2016) maternally fed on a high fat diet.
It is known that mitochondria are involved in production of free radical as well as play a great role in oocyte development via release of Ca2 + signaling, and the production of ATP and reactive oxygen species (Dumollard et al., 2007). Consequently, ascorbate is known concentrated in granulosa cells, theca cells, luteal cells, and the oocyte (Musicik et al., 1996 ; Zreik et al., 1999). Abnormal depletion of catalase, SOD and glutathione-S-transferase facilitated increase of the reactive oxygen species (Aiken et al., 2016) and aldehydic products of lipid (Tjalkens et al., 1998) leading to cell damage. (Kemp et al., 2008).
Lipotoxicity is characterized by increase production of circulating long-chain saturated fatty acids from either the adipocytes or the diet (Szendroedi and Roden, 2009) , which enhance the production of reactive oxygen species with subsequent mitochondrial dysfunction and endoplasmic reticulum stress (Schaffer, 2003; Luzzo et al., 2012). Also, consumption of high-fat diet abnormally decreased the mitochondrial membrane potential in both immature and mature oocytes (Fujii and Funahashi, 2009; Mitchell et al., 2009; Kanaya et al., 2007). These was involved in depletion of the ATP associated decrease of the oxidative phosphorylation, liberation of oxygen free radical and activation of caspases leading to DNA fragmentation and development of follicular atresia (Tamura et al., 2008; Wu et al., 2011; Yang et al., 2012).
The mammalian reproductive cycles of offspring are tightly regulated by the hypothalamus (gonadotrophin-releasing hormone)-pituitary (follicle-stimulating hormone) and ovarian axis (17β- estradiol) (Nett et al., 2002; Kermath and Gore, 2012). AMH is a glycoprotein secreted by granulosa cells in the developing ovarian follicle (Hagen et al., 2010). Both AMH, FSH represent an indicator of both normal and abnormal ovarian function. It is known that follicle stimulating hormones promoted de novo biosynthesis of steroid hormone from the circulating lipoprotein and cholesterol in the theca and granulosa cells (Miller and Auchus, 2011).
The observed alterations of assayed maternal hormones reflected the damage of granulosa cells which intern disturpted the hypothalamic-pituitary-ovarian axis leading to altered ovarian hormonal secretion. These were assessed by the increase of maternal serum levels of both estradiol level parallel with depletion of both follicle stimulating hormone and anti-Mullerian hormone.
The present findings agree with the work of Lee et al.(2016) in obese girl and Skaznik-Wikiel et al.(2016) and on experimental rat (Ambrosetti et al., 2016) and mice offspring (Lin et al., 2017) on mice fed on a high fat diet.
In contrast, experimental hypercholesterolemic groups supplemented diet containing barley and/or dates, exhibited a moderate improvement of the ovary structures. The ovaries restored a large numbers of ovarian follicles especially in those maternally fed on hypercholesterolemic diet containing barley. The ovarian stroma possessed improved fibroblast infiltrated by thin connective tissue. The antioxidant enzymes catalase, superoxide dismutase and glutathione s transferase restored the almost normal value coincides with decreased malondialdhyde manifesting decreased lipid peroxidation. Also, the maternal serum levels of estradiol, follicle stimulating hormone and anti-mullerian hormone were improved reflected the ameliorated cytological structure of oocyte and follicle cells.
The capacity of improvement of ovarian follicle structure and function in studied group fed on hypercholesterolemic diet and barley attributed to different phytonutrients in barley such as β-glucans which enhanced excretion of bile acids (Ellegard and Andersson, 2007; Abumweis et al., 2010) activating secretion of cholesterol 7a-hydroxylase, which facilitated cholesterol elimination in the body (Nilsson et al 2007 and Daou and Zhang, 2012) as well inhibit tumor necrosis factor-a, the marker of inflammation (Sener et al., 2006) and scavenging the ROS, the premotor of DNA damage (Oliveira et al., 2014).
The ovaries of offspring maternally fed on hypercholesterolemic diet containing date showed moderate improvement but of least degree comparing to that containing barley and date or barley alone. Although, the date palm fruits are rich in dietary fibers (Vinita and Punia, 2016) and phenolic antioxidants (Ghnimi et al.2016) and trace elements such as zinc, selenium (Singh et al., 2012) and vitamins A, B complex and C (Vayalil, 2012), it is rich in sugar (44-88%) (Al-Shahib and Marshall, 2003).
Maternal feeding on high cholesterol diet showed altered blood sugar level characteristic with type 2 diabetes (El-Sayyad et al.,2010, 2011, 2014). Supplementation of date palm fruits beside high cholesterol diet altered the blood glucose level and over helm its antioxidant activity which resoluted its capacity of improving the ovarian tissues assessed in histo-cytological structures , alterations of the antioxidant enzymes and hormonal level.
The authors finally concluded that offspring maternally fed on hypercholesterolemic diet developed reduction of ovarian follicular reserve, increases lipid peroxidation and altered maternal reproductive hormone involved in ovarian development. These dramatic alterations were improved post adding barley and/or date palm fruits to the hypercholesterolemic diet.
References
- Ahmadizar, F., Souverein, P., de Boer, A., Maitland-van der Zee, A.H. Under-treatment of hypertension and hypercholesterolemia in children and adolescents with type 1 diabetes- Long term follow-up on time trends in the occurrence of cardiovascular disease, risk factors and medications use.Br J Clin Pharmacol. 2018; 2018 Apr;84(4):776-785.
- AbuMweis, S.S., Jew, S. and Ames, N.P., Beta-glucan from barley and its lipid lowering capacity: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr.2010; 64 (12):1472-1480.
- Aiken, C.E., Tarry-Adkins, J.L., Penfold, N.C., Dearden, L. and Ozanne, S.E..Decreased ovarian reserve, dysregulation of mitochondrial biogenesis , and increased lipid peroxidation, in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 2016;30(4):1548-56.
- Al-Safi, Z.A., Polotsky, A., Chosich, J., Roth, L., Allshouse, A.A., Bradford, A.P.and Santoro, N. Evidence for disruption of normal circadian cortisol rhythm in women with obesity. Gynecol Endocrinol.2017 ; 25:1-5.
- Al-Shahib, W.and Marshall, J.. The fruit of the date palm: its possible use as the best food for the future?. Int J Food Sci Nutr. 2003 ;54(4):247-59.
- Ambrosetti, V., Guerra, M., Ramírez, L.A., Reyes, A., Álvarez, D., Olguín, S., González-Mañan, D., Fernandois, D., Sotomayor-Zárate, R., Cruz, G. Increase in endogenous estradiol in the progeny of obese rats is associated with precocious puberty and altered follicular development in adulthood. Endocrine. 2016. ;53(1):258-70.
- Baek, A.E., Yu, Y.A., He, S., Wardell, S.E., Chang, C.Y., Kwon, S., Pillai, R.V., McDowell, H.B., Thompson, J.W., Dubois, L.G., Sullivan, P.M., Kemper, J.K., Gunn, M.D., McDonnell, D.P. and Nelson, E.R,. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancermetastasis through its actions on immune cells. Nat Commun. 2017;8(1):864.
- Bock, P., Karmer, R. and Paverka, M., A simple assay for catalase determination. Cell Biol Monogr 1980; 7:44-74.
- Carvalho, D.O., Curto, A.F., Guido, L.F. Determination of Phenolic Content in Different Barley Varieties and Corresponding Malts by Liquid Chromatography-diode Array Detection-Electrospray Ionization Tandem Mass Spectrometry. Segura-Carretero A, Arráez-Román D, eds. Antioxidants. 2015.;4(3):563-576.
- Češka, R., Hyperlipoprotienemias and (not only) atherosclerosis: fragments from history and present. Cas Lek Cesk. 2017;156(6):303-307.
- Cheong, Y., Sadek, K.H., Bruce, K.D., Macklon, N., Cagampang, F.R., Diet-induced maternal obesity alters ovarian morphology and gene expression in the adult mouse offspring. Fertil Steril. 2014;102(3):899-907.
- Czubkowski, P., Wierzbicka, A., Pawłowska, J., Jankowska, I., Socha, P., Obesity, lipid profiles and oxidative stress in children after liver transplantation. Acta Biochim Pol. 2017; 64(4):661-665.
- Daou ,C. and Zhang, H., Oat beta-glucan: its role in health promotion and prevention of Comp Rev Food Sci Food Safety 2012; 11(4):355-365.
- Desai, M., Jellyman, J.K., Han, G., Beall, M., Lane, R.H., Ross, M.G., Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obstet Gynecol.2014;211(3):237.e1-237.e13.
- Dumolt JH, Radhakrishnan SK, Moghadasian MH, Le K, Patel MS, Browne RW, Rideout TC. . Maternalhypercholesterolemia enhances oxysterol concentration in mothers and newly weaned offspring but is attenuated by maternal phytosterol supplementation. J Nutr Biochem. 2018;52:10-17.
- Dumollard, R., Duchen, M. and Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol.2007; 77:21-49.
- Dvořáková, M., Guido, L.F., Dostálek, P., Skulilová, Z., Moreira, M.M., Barros, A.A. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. Inst. Brew. 2008.;114:27–33.
- Ellegard, L and Andersson, H., Oat bran rapidly increases bile acid excretion and bile acid synthesis: an ileostomy study. Eur J Clin Nutr.2007; 61:938-945.
- El-Sayyad, H.I.H., Amoura, M.A., Gadallah, A.A., Bakr, I.H., Protective effects of Allium sativum against defects of hypercholesterolemia on pregnant rats and their offspring. Int J Clin Exp Med 2010;3(2):152-163.
- El-Sayyad, H.I.H, El-Sherbiny, M., Sobh, M.A., Abou-El-Naga, A.M., Ibrahim, M.A.N. and Mousa, S.A., Protective effects of Morus alba leaves extract on ocular functions of pups from diabetic and hypercholesterolemic mother rats. J.Biol.Sci. S.A.; 7 : 715-728.
- El-Sayyad, HIH , Al-Haggar, M.M.S., El-Ghawet, H.A., Bakr, I.H.M.,.Effect of maternal diabetes and hypercholesterolemia on fetal liver of albino Wistar rats. Nutrition 2014; 30 (2014) 326–336.
- Enkhmaa, B., Shiwaku, K., Katsube, T., Kitajima, K., Anuurad, E., Yamasaki, M. Mulberry (Morus alba L.) leaves and their major flavonol quercetin (6 malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice. J Nutr ;135:729–34.
- Englich, B., Herberth, G., Rolle-Kampczyk, U., Trump, S., Röder, S., Borte ,M., Stangl, G.I., von Bergen, M., Lehmann, I., Junge, K.M., Maternal cytokine status may prime the metabolic profile and increase risk of obesity in children. Int J Obes (Lond).2017;41(9):1440-1446.
- Fujii, W. and Funahashi, H., Exogenous adenosine reduces the mitochondrial membrane potential of murine oocytes during the latter half of in vitro maturation and pronuclear formation following chemical activation. J Reprod Dev.2009; 55:187–193.
- Ghnimi, S., Umer, S., Karim, A. and Kamal-Eldin, A., . Date fruit (Phoenix dactylifera): An underutilized food seeking industrial valorization. NFS J 6 (2017) : 1-10.
- Habig ,W.H., Pabst, M.J. and Jakoby, W.B., Glutathione S-transferases: the first enzyrnatic step in rnercapturic acid formation. J Biol Chem.1974; 249:7130-7139.
- Hagen, C.P., Aksglaede, L., Sørensen, K., Main, K.M., Boas, M., Cleemann, L., Holm, K., Gravholt, C.H., Andersson, A.M., Pedersen, A.T., Petersen, J.H., Linneberg, A., Kjaergaard, S.and Juul, A. ,Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010; 95(11):5003-10.
- Hall, M.C. The effect of oxysterols, individually and as a representative mixture from food, on in vitro cultured bovine ovarian granulosa cells. Mol Cell Biochem. 2006; 292(1-2):1-11.
- Hamad, I., Abdelgawad, H., Al, J.S., Zinta, G., Asard, H., et al. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) from Saudi Arabia to assess their quality. Molecules 2015; 20: 13620-13641
- Heydemann, A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J Diabetes Res.2016;2016:2902351.
- Hu, J., Zhang, Z., Shen, W.J. and Azhar, S., Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond).2010; 7:47.
- Ighodaro OM, Akinloye OA.First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018; 54(4):287-293.
- Idehen, E., Tang, Y., Sang, S. Bioactive phytochemicals in barley.J Food Drug Anal. 2017; 25(1):148-161.
- Ingvorsen, C., Brix, S., Ozanne, S.E., Hellgren, L.I.., The effect of maternal Inflammation on foetal programming of metabolic disease. Acta Physiol (Oxf).2015;214(4):440-9.
- Kajihara, T., Uchino, S., Suzuki, M., Itakura, A., Brosens, J.J. and Ishihara, O. , Increased ovarian follicle atresia in obese Zuker rats is associated with enhanced expression of the forkhead transcription factor FOXO1. Med Mol Morphol.2009 ;42(4):216-221.
- Kalyani, M., Hasselfeld, K., Janik, J.M., Callahan, P. and Shi, H., Effects of High-Fat Diet on Stress Response in Male and Female Wildtype and Prolactin Knockout Mice. PLoS ONE 2016;11(11): e0166416.
- Kanaya, H., Hashimoto, S., Teramura,T., Morimoto, Y., Matsumoto, K., Saeki ,K., Iritani, A. and Hosoi, Y., Mitochondrial dysfunction of in vitro grown rabbit oocytes results in preimplantation embryo arrest after activation. J Reprod Dev.2007; 53:631–637.
- Kalra,, Jood, S. Effect of dietary barley β-glucan on cholesterol and lipoprotein fractions in rat. J Cereal Sci, 2000.; 31(2) 141-145.
- Kemp, M., Go, Y.M. and Jones. D.P., Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med.2008; 44: 921-937.
- Kermath, B.A. and Gore, A.C., Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinology 2012; 96(1):1-12.
- Lee, H.S., Yoon, J.S. and Hwang, J.S.,Luteinizing Hormone Secretion during Gonadotropin-Releasing Hormone Stimulation Tests in Obese Girls with Central Precocious Puberty. J Clin Res Pediatr Endocrinol.2016;8(4):392-398.
- Léveillé, P., Tarrade, A., Dupont, C., Larcher, T., Dahirel, M., Poumerol, E., Cordier, A.G., Picone, O., Mandon-Pepin, B., Jolivet, G., Lévy, R. and Chavatte-Palmer, P., Maternal high-fat diet induces follicular atresia but does not affect fertility in adult rabbit offspring. J Dev Orig Health Dis.2014;5(2):88-97.
- Li X, Kang X, Deng Q, Cai J, Wang Z. Combination of a GnRH agonist with an antagonist prevents flare-up effects and protects primordial ovarian follicles in the rat ovary from cisplatin-induced toxicity: a controlled experimental animal study. Reprod Biol Endocrinol 2013;11:16.
- Lin, Y.J., Tsai, C.C., Huang, L.T., Sheen, J.M., Tiao, M.M., Yu, H.R., Chen, C.C. and Tain, Y.L., Detrimental effect of maternal and post-weaning high-fat diet on the reproductive function in the adult female offspring rat: roles of insulin like growth factor 2 and the ovarian circadian clock. J Assist Reprod Genet.2017;34(6):817-826.
- Luzzo, K.M., Wang, Q., Purcell, S.H., Chi, M., Jimenez, P.T., Grindler, N., Schedl, T. and Moley, K.H. ,High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One.2012;7(11):e49217.
- McCord, J.M., Superoxide dismutase, lipid peroxidation, and bell- shaped dose response curves. Dose Response.2008;6(3):223-38.
- McRorie, J.W., McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J Acad Nutr Diet.;117(2):251-264.
- Miller, W.L. and Auchus, R.J., The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev.2011; 32: 81-151.
- Mitchell, M., Schulz, S.L., Armstrong, D.T. and Lane, M.,). Metabolic and mitochondrial dysfunction in early mouse embryos following maternal dietary protein intervention. Biol Reprod.2009; 80:622–630.
- Musicki, B., Kodaman, P.H., Aten, R.F. and Behrman, H.R., Endocrine regulation of ascorbic acid transport and secretion in luteal cells. Biol Reprod.1996; 54: 399 406.
- Nett, T.M., Turzillo, A.M., Baratta, M. and Rispoli, L.A., Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol.2002; 23(1-2):33-42.
- Newman, R.K., Newman, C.W. (2008). Barley for food and health: science, technology, and products. A John Willey & Sons, INC., Publication.
- Nilsson, L.M., Abrahamsson, A., Sahlin, S., Gustafsson, U., Angelin, B., Parini, P. and Einarsson, C., Bile acids and lipoprotein metabolism: effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem Biophys Res Commun.2007; 357(3):707-711.
- Nishikimi, M., Appaji, N. and Yagi, K., The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972; 46:849-854.
- Noor, R., Mittal, S. and J., Superoxide dismutase–applications and relevance to human diseases. Med Sci Monit.2002;8(9):RA210-5.
- Ohkawa, H., Wakatsuki, A. and Kaneda, C., Assay for lipid peroxides in animals tissues by thiobarbituric acid reaction. Anal Biochem1982.; 95:351-358.
- Oliveira, R.J., Pesarini, J.R., Salles, M.J.S., Kanno, T.Y.N., Lourenço, A.C., Leite, V., da Silva, A.F., Matiazi, H.J., Ribeiro, L.R. and Mário Sérgio Mantovani, M.S., Effects of β -glucan polysaccharide revealed by the dominant lethal assay and micronucleus assays, and reproductive performance of male mice exposed to cyclophosphamide. Genet Mol Biol.2014; 37(1):111-119.
- Ramalho, L., da Jornada, M.N., Antunes, L.C. and Hidalgo, M.P., Metabolic disturbances due to a high-fat diet in a non-insulin-resistant animal model. Nutr Diabetes.2017;7(3):e245.
- Rosmond, R. and Björntorp, P. ,Low cortisol production in chronic stress. The connection stress-somatic disease is a challenge for future research. Lakartidningen. 2000 20;97(38):4120-4
- Schaffer, J.E., Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003; 14(3):281–287.
- Sener, G., Eksioglu-Derniralp, E., Cetiner, M., Ercan, F. and Yegen, B., ß-glucan ameliorates methotrexate-induced oxidative organ injury via its antioxidant and immune-modulatory effects. Eur J Pharmacol. 2006; 542 (1-3):170-178.
- Singh, V., Guizani, N., Essa, M.M., Hakkim, F.L. and Rahman, M.S., Comparative analysis of total phenolics, flavonoid content and antioxidant profile of different date varieties (Phoenix dactylifera L.) from Sultanate of Oman. Int Food Res J.2012; 19:1063-1070.
- Skaznik-Wikiel, M.E., Swindle, D.C., Allshouse, A.A., Polotsky, A.J. and McManaman, J.L. ,High-fat Diet causes subfertility and compromised ovarian function independent of obesity in mice. Biol Reprod. 2016;94(5):108.
- Szendroedi,J. and Roden,, Ectopic lipids and organ function.Curr Opin Lipidol.2009 ;20(1):50-6.
- Tamura, H., Takasaki, A., Miwa, I., Taniguchi, K., Maekawa, R., Asada, H., Taketani, T., Matsuoka, A., Yamagata ,Y., Shimamura, K., Morioka, H., Ishikawa, H., Reiter, R.J. and Sugino, N.,. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res.2008; 44(3):280–287.
- Tjalkens, R.B., Valerio, L.G., and Awasthi, Y.C. andPetersen, D.R. Association of glutathione S-transferase isozyme-specific induction and lipidperoxidation in two inbred strains of mice subjected to chronic dietary iron overload. Toxicol Appl Pharmacol.1998 l;151(1):174-81.
- Tsoulis, M.W., Chang, P.E., Moore, C.J., Chan, K.A., Gohir, W., Petrik, J.J., Vickers, M.H., Connor, K.L. and Sloboda, D.M. ,Maternal High-Fat Diet-Induced Loss of Fetal Oocytes Is Associated with Compromised Follicle Growth in Adult Rat Offspring. Biol Reprod. 2016;94(4):94.
- Vayalil, P.K. Date fruits (Phoenix dactylifera Linn): an emerging medicinal food. Crit Rev Food Sci Nutr. 2012; 52:249–271.
- Vinita, V. and Punia, D., Nutritional composition of fruit of four date palm (Phoenix dactylifera L.) cultivars grown in Haryana, India. Asian J. Dairy & Food Res, 35 (4) (2016) : 331-334.
- Wang, Y., Harding, S.V., Thandapilly, S.J., Tosh, S.M., Jones, P.J.H., Ames, N.P. Barley β-glucan reduces blood cholesterollevels via interrupting bile acid metabolism. Br J Nutr.;118(10):822-829.
- Winzell, M.S., Ahrén, B., The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. 2004;53 Suppl 3:S215-9.
- Wu, L.L., Norman, R.J. and Robker, R.L. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Repro Fertil Dev 2011; 24(1):29–34.
- Wu, L.L/Y/, Dunning, K.R., Yang, X., Russell, D.L., Lane, M., Norman, R.J and Robker,R.L. High-fat diet causes lipotoxicity responses in cumulus oocyte complexes and decreased fertilization rates. Endocrinology 2010;151:5438-5445.
- Wu, L.L., Russell, D.L., Wong, S.L., Chen, M., Tsai,T.S., St John, J.C.,Norman, R.J., Febbraio, M.A., Carroll, J., Robker, R.L., Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development2015 142: 681-691.
- Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma T, Zheng W, Sun R, Shen W, Sha J, Cooke HJ, Shi Q. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS One. 2011;6(1):e16046.
- Xu, M., Che, L., Yang, Z., Zhang, P., Shi, J., Li, J., Lin, Y., Fang, Z., Che, L, Feng, B., Wu, D., Xu, S., Effect of high fat dietary intake during maternal gestation on offspring ovarian health in a pig model. Nutrients2016, 8, 498.
- Yang, X., Wu, L.L., Chura, L.R., Liang, X., Lane M, Norman, R.J. and Robker R., Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012; 97(6):1438–1443.
- Zhou, Z., Lin, Q., Xu, X., Illahi, G.S., Dong, C., Wu, X.,.Maternal high- fat diet impairs follicular development of offspring through intra ovarian kisspeptin/GPR54 system. Reprod Biol Endocrinol.2019;17(1):13.
- Zreik, T.G., Kodaman, P.H., Jones, E.E., Olive, D.L, and Behrman, H.R.. Identification and characterization of an ascorbic acid transporter in human granulosa-lutein cells. Mol Hum Reprod 1999.; 5: 299-302.

This work is licensed under a Creative Commons Attribution 4.0 International License.





