Manuscript accepted on : 17-Sep-2018
Published online on: 09-10-2018
Plagiarism Check: Yes
Guetouache Mourad1,2 and Guessas Bettache2,3
and Guessas Bettache2,3
1Department of Microbiology and Biochemistry, Faculty of Science, University Mohamed Bouadiaf of M'sila, 28 000, Algeria.
2Department of Biology, Faculty of Sciences, University 1 Ahmed Benbella of Oran, Algeria.
3Department of Biology, Faculty of Sciences, Laboratory of Applied Microbiology, University 1 Ahmed Benbella of Oran, Algeria.
Corresponding Author E-mail: mouradeg33@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2683
ABSTRACT: Morphological, physiological and biochemical characteristics were employed to identify lactic acid bacteria (LAB), isolated from traditional (butter) was collected from different rural areas of the province of Djelfa. Among 177 isolates, 79 lactic acid bacterial (LAB) strains were isolated and purified. The results obtained show that the isolates obtained belong to the following genus Lactobacillus, Lactococcus, Enterococci and Leuconostoc characterize the biodiversity of this traditional butter studied. Only Gram-positive and catalase negative isolates were identified at species level. The most common LAB belonging to the species Lactobacillus alimentarius (15.19 %), Lactobacillus plantarum (22.78 %), Lactobacillus fermentum (18.99 %), Lactobacillus brevis (06.33 %), Lactococcus lactis (12.66 %), Lactococcus cremoris (06.33 %), Leuconostoc mesenteroides (06.33 %) and Enterococcus faecalis (11.39 %). The samples pH average was 6.06 ± 0.34, microbiological analysis results were; total mesophilic aerobic flora (TMAF) (2, 22 ± 0, 68).10 3cfu/ml, total coliforms 0,54 ± 0.56 ufc/ml, fecal coliforms 0,6 ± 0.50 cfu/ml, yeast (0,48 ± 0.31). 10 cfu/ml, Staphylococcus aureus, Salmonella and moulds weren’t detected.
KEYWORDS: Acid Lactic Bacteria; Antimicrobial; Butter; Identification; Isolation; Proteolytic
Download this article as:| Copy the following to cite this article: Mourad G Bettache G. Characterization of Lactic Acid Bacteria Isolated from Traditional Butter Produced in Djelfa Province of Algeria. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Mourad G Bettache G. Characterization of Lactic Acid Bacteria Isolated from Traditional Butter Produced in Djelfa Province of Algeria. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31442 |
Introduction
Fermented milk is a dairy product provides the human diet with nutritious compounds of varied flavors, aromas, and textures. These which product is based on the metabolic activity of lactic acid bacteria to ferment sugars, especially glucose and galactose, so to produce lactic acid and aroma substances that give typical flavors and tastes to fermented products. Several types of fermented milk products have been reported to exist throughout the world. The most popular of them in North African are Jben, Lben, Klila and Raib (Mechai and Kiran, 2008). The name “cheese” is reserved to fermented product or not obtained by coagulating milk, cream, skim milk, or a mixture thereof, followed by draining. The cheese is made either by the traditional method in the rural environment and traditional or by the method semi industrial or industrial methods which remains limited (Rhiat et al., 2013). In Algeria, many traditional dairy products are not identified and studied; several types of traditional dairy are classified and identified in different parts of our country. Among these different types, we mention the following names Butter, Smen, Mechouna, Bouhezza, Madeghissa, Klila, Jben Takammerite, Aoules, Igounanes and Takammerite (Guetouache and Guessas, 2015),The fresh butter is obtained after churning the fermented milk (Raib).
The latter is occasionally increased by a quantity of warm water (40 to 50 °C) at the end of churning to promote the agglomeration of lipid globules and increase the yield of Butter. A perforated spoon separates the fat globules appearing on the surface, after churning, the fresh butter obtained has a soft consistency due to the high concentration of water. The excess butter produced is processed into rancid butter (Smen) through the washing of fresh butter with warm water, brining, salting (8-10g / 100g) and conditioning (Hadj Aissa, 2011).
The lactic acid bacteria (LAB) may be defined as a group of Gram-positive, nonsporingcocci and rods with nonaerobic habit but aerotolerant, which produce lactic acid as the majorend product during fermentation of carbohydrates(Halasz, 2009). Lactic acid bacteria include various major genera: Lactobacillus, Lactococcus, Carnobacterium, Enterococcus, Lactosphaera, Leuconostoc, Melissococcus, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus and Weissella. Other genera are: Aerococcus Microbacterium, Propionibacterium an Bifidobacterium (Carr et al, 2002; Parada et al, 2007). Many strains of LAB are among the most important groups of microorganisms used in the food and feed industries. LAB have been used in food preservation and for the modification of the organoleptic characteristics of foods, for example flavors and texture. Various strains of LAB can be found in dairy products fermented, meats, fermented vegetables, sourdough bread, etc.
The European Food Safety Authority (EFSA) has stated that several LAB strains can be considered to have “Qualified Presumption of Safety” QPS-status. Moreover, nowadays, LAB play an important role in the industry for the synthesis of chemicals, pharmaceuticals, or other useful products. Also, the biotechnological production of lactic acid has recently reported that offers a solution to the environmental pollution by the petrochemical industry (Paneri, 2013). The purposes of this study were the isolation and taxonomic determination of large number lactic acid bacteria from traditional dairy products (Butter) and characterization of different groups of lactic acid bacteria using classical methods.
Material and Methods
Rural Area Study
The wilaya of Djelfa is located in the central part of northern Algeria (Figure 1). This centrality allows the wilaya to develop more and more. It represents the perfect link from North to South of the country and from east to west and an undisputed point of passage. Due to the conditions of its natural environment and the extent of its territory, the Wilaya of Djelfa is a steppic Wilaya where sheep farming predominates, its main vocation is pastoral (NAID, 2014).
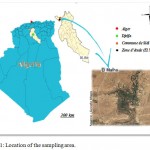 |
Figure 1: Location of the sampling area.
|
Samples Collection
Five samples of Butter were collected from the rural area (El Malha) of Djelfa province, Algeria. Samples were brought to the laboratory at 4-5°C by using of an icebox, stored in laboratory under refrigeration at 4°C, and analyzed immediately within 24 hours. the pH measurement of the samples is performed by a pH meter with an Orion Research type combination electrode and previously calibrated with buffer solutions at pH 4 and pH 7.
Microbiological Analysis
Microbiological analysis is performed for Butter to search: total aerobic mesophilic flora (FMAT) is enumerated on PCA agar (Plate Count Agar) incubated for 24 h at 30°C. Total coliforms, faecal coliforms and Escherichia coli were estimated by a three tube most probable number (MPN) technique. Enumeration and isolation of Staphylococcus aureus, was carriedout by surface plating technique onto Baird Parkeragar (Meshref, 2010). For Salmonella, there is provided a pre-enrichment on selenite-cysteine medium for 12 hours at 37°C, followed by an enrichment on bouillon of tetrathionate for 24 hours at 37°C, then the enumeration and isolation were carried out on SS medium (Salmonella-Shigella) after 24 hours of incubation at 37°C. The sulphitoreductor-clostridia are counted in the culture medium reinforced Clostridium Agar in tubes to promote anaerobic conditions, with thermic treatment for 10 minutes at 80°C to activate the spores of clostridia: they can persist in a latent form in milk, germinate as soon as conditions are favorable and secrete toxic substances. The tubes are incubated for 48 h at 37°C. Only black colonies are counted. The microbiological analysis is performed in three steps: preparation of dilutions, seeding in the culture medium and enumeration of microorganisms (Rhiat et al., 2013).
Study of lactic micro-flora
Ten grams of butter was homogenized with 90 ml sterile NaCl solution (0.85%, w/v) to a homogenous suspension and then a tenfold serial dilution in NaCl solution (0.85% w/v) (Guessas et al 2012). Enumeration of (LAB) was determined using various elective media (Table 1). After appropriate incubation time, plates containing 25 to 250 colonies were enumerated and recorded as colony forming units (cfu) per ml of culture sample (Ashraf and Smith, 2015). Repeated streaking one appropriate agar media (Khedid et al., 2009) purified the selected colonies. In different conditions including at 4°C for MRS, M17 and MSE plates and at -20°C for broths MRS, M17 and MSE supplemented by 20 % glycerol for further use (Mathara et al., 2004).
Table 1: Media and condition for enumeration and isolation of lactic micro-flora (Khedid et a.l, 2009).
| Genus | Media | T °C | Duration (h) | Incubation |
| Enterococcus | M17 | 45 | 48 | Aerobic |
| Lactococcus | Elliker | 30 | 48 | Aerobic |
| Leuconostoc | MSE | 21 | 72 – 144 | Aerobic |
| Pediococcus | MRS | 30 | 48 | Aerobic |
| Mesophilic Lactobacillus | MRS | 30 | 24 – 48 | Aerobic |
| Thermophilic Lactobacillus | MRS | 45 | 24 – 48 | Aerobic |
All isolates were examined for Gram reaction, production of catalase, and oxidase activity. Gram-positive and catalase- and oxidase-negative isolates were stored for further analyses. Purification of the isolates was done by repeated pour plating technique using the same agar medium until pure cultures were obtained. Pure cultures were transferred and maintained in different agar. Duplicate tubes of the isolates were prepared, one tube was stored in refrigerator as stock culture, and the other tube was used for identification studies (Neti and Erlinda, 2011).Isolates were identified using the following tests: ammonia production from arginine, CO2 production from glucose, and growth at different temperatures (4, 8, 10, 15 and 45°C), growth at different pH values, and growth at different NaCl concentrations (Schillinger and Lucke, 1989). Each strain under examination was sub-cultured twice overnight in MRS broth. All strains were initially tested for Gram reaction, catalase production and spore formation. Cell morphology and colony characteristics on MRS agar were also examined, and a separation into phenotypic groups was undertaken. Only the Gram-positive, catalase-negative isolates were further identified. Growth at different temperatures was observed in MRS broth after incubation for 5 days at 10°C, 15°C, 37°C, 15°C and 45°C. Hydrolysis of arginine was tested in M16BPC. Growth in the presence of 2, 4, 10 and 6.5 % NaCl performed in MRS broth for 5 days. Utilization of citrate was realized in Kempler and Mc Kay (1980) medium. Production of acetone from glucose was determined using Voges-Proskauer test. For performing the biochemical tests, an MRS-BCP broth medium (BCP 0.17 g/l) was used. The carbon source was added to the sterile basal medium as filter sterilized solution to a final concentration of 1 %. Carbohydrates utilization was assessed at the 24 and 48th h (Guetouache and Guessas, 2015). All strains were tested for fermentation of the following twenty sugars: Arabinose, Xylose, Galactose, Fructose, Mannitol, Sorbitol, Cellubiose, Maltose, Lactose, Melibiose, Saccharose, Trehalose, Esculin, Manose, Rhamnose, Ribose, Sucrose,and Raffinose; to ensure anaerobic conditions, two drops of sterile paraffin oil were placed in each tube after inoculation (Carr et al., 2002).
Technological Study of LAB
Acidification properties of the (LAB) were measured by the change in pH with time. The strains were initially grown in MRS broth and then in sterile skim milk (01 %) supplemented with yeast extract (0.3 %), were inoculated with overnight cultures, which had been previously activated by two successive transfers in milk. The pH changes were measured with a pH meter after 6, 12, and 24 h of incubation at 30°C. Coagulation of milk was determined after 24 h of incubation at 30°C. Acidification activity was measured by following the change in the pH during time according to the method described by Accolas et al (1977), using NaOH (N/9) in the presence of phenolphthalein indicator (1 % in alcohol). Samples were inoculated on MRS broth containing 1% (w/v) skimmed milk, incubated under anaerobic conditions at 37±1°C for 48h (Marroki et al., 2011). The proteolytic LAB strains were tested using MRS agar containing 2 % (w/v). Petri dishes were incubated at 37 °C for three days and observed daily, the proteolytic LAB strains were identified by the presence of clear zone around the colonies. The radius of the halo formation (in mm) at the end of incubation was measured (Jini et al., 2011). As for the proteolytic activity residual proteins concentrations in the culture were estimated by a Coomassie G-250 binding procedure (Bradford, 1976). The isolates of LAB that exhibited proteolytic and characterized by different biochemical tests were further tested for their antibacterial activity against different pathogens. The indicator strains included, Bacillus subtilis ATCC 93 72, Bacillus cereus ATCC 10876, Staphylococcus aureus ATCC 65 38 and Escherichia coli ATCC, L 25 922, were obtained from the Educational Laboratory of the M’sila University. His agar-well diffusion method was employed in the screening of LAB for antimicrobial activities. Indicator lawns were prepared by inoculating 20 ml of BHI molten agar media with 100 μl of an overnight culture of each indicator organism and allowing them to solidify in a Petri dish. Wells were cut into the agar with a sterile 6 mm diameter cork-borer and sealed with two drops of sterile agar. Fifty micro-liters (50 μl) of the filtered cell-free supernatant of test strains was separately placed into the wells. The plates, prepared in duplicate, were kept at 4°C for 24 h into the agar and then incubated at 37°C for 24 h. They were then observed for possible clearing of zones (inhibition zones). The antimicrobial activity was determined by measuring the diameter of the inhibition zones around the well using caliper in mm. Results were recorded as no inhibition (−), weak inhibition (+), moderate inhibition (++), and strong inhibition (+++) when the diameter is less than 1–4 mm, better than 4–8 mm, and better than 8–12 mm, respectively (Akabanda et al., 2014).
Statistical Analysis
Average colony forming units of microbial load was calculated using descriptive statistics of spread sheet Microsoft excel.
Results and Discussion
Physicochemical and Microbiological Analysis
The results of physicochemical analysis were shown in Table 2. Where pH range for Butter was 05.60 to 05.4 with an average of 06.06 ± 00.34 these values are similar to those found by Keyvani, 2015.
Table 2: pH of Buttre samples.
| Samples | |||||
| S1 | S2 | S3 | S4 | S5 | |
| pH | 5.6 | 5.8 | 6.2 | 6.3 | 6.4 |
| pH (M ± SD) | 6.06 ± 0.34 | ||||
S: Samples, M: Mean, SD: Standard deviation.
Effective cleaning procedures, including removing faecal material from udders prior to milking and good manufacturing practices during the manufacture of traditional dairy products can reduce the risk (Guetouache and Guessas, 2015 ), consumers’ requirements for traditional fermented dairy food products are generally increased due to their proved gastronomic quality and positive effects on human health. However, the tightened legislation on food safety results in lower production flexibility, homogeneity in the food production and in the loss of food diversity and traditional specificity. Hence, the preparation of traditional dairy products using the standardized traditional technology is crucial (Terzic-Vidojevic et al., 2014).The results of some microbiological properties of Buttre are presented in Table 3. The total aerobic mesophilic bacteria (TAMB) counts ranged from 102 x 104 cfu/ml and 2.4 x 104 cfu/ml with the average of (2,22 ± 0.68) x 104cfu/ml, the total coliforms counts ranged from 0.1 cfu/ml and 1.2 cfu/ml with the average count of coliforms bacteria was 0,54 ± 0.56 cfu/ml, the fecal coliforms counts ranged from 0.2 cfu/ml and 1.2 cfu/ml with the average count of coliforms bacteria was 00,6 ± 0.50 cfu/ml and pathogenic bacteria Staphylococcus and Salmonella were not detected, the average yeasts counts determined was from (0,48 ± 0.31) x 10cfu/g. According to the results obtained, in this research, the counts TAMB, total coliforms bacteria, fecal coliforms bacteria and presence yeasts in traditional Butter were higher than the upper limits given in European Commission (EC, 2001). It is not surprising to obtain high microbiological counts in traditional products with artisanal manufacturing methods due to the use of unpasteurized milk.
Table 3: Results of Microbiological analysis (cfu / ml) of Butter.
| Samples | |||||||
| S1 | S2 | S3 | S4 | S5 | M ± SD | Norm | |
| Yeast. 10 | 0.3 | 0.2 | 1 | 0.5 | 0.4 | 0.48 ± 0.31 | 102/g [41] |
| Total aerobic mesophilic. 104 | 2.3 | 2.1 | 3.1 | 2.4 | 1.2 | 2.22 ± 0.68 | 105 /g [41] |
| Total coliforms | 0.1 | 0.2 | 1.1 | 0.1 | 1.2 | 0.54 ± 0.56 | 10/g [41] |
| Fecal coliforms. | 1.1 | 0.2 | 0.3 | 0.2 | 1.2 | 0.6 ± 0.50 | 10/g [41] |
| Staphylococcus aureus. 103 | Abs | Abs | Abs | Abs | Abs | Abs | Abs/1g [41] |
| Salmonella. 103 | Abs | Abs | Abs | Abs | Abs | Abs | Abs/1g [41] |
S: Samples, M: Mean, SD: Standard deviation, Abs: Absence,
Isolation and Identification of LAB
The enumeration of the lactic flora on MRS, M17, MSE and Elliker gives respective mean values of 05.8.x 104cfu/ml, 03.44 x 103cfu/ml, 01.22 x 102cfu/ml and 04.49 x 102 cfu/ ml respectively. The characteristics of LAB are shown in Tables 4 and 5. All isolates were Gram-positive and catalase- negative bacteria. Seventy-nine LAB strains were isolated and purified.
This study shows that the biodiversity of traditional Butter studied is characterized by the species, Lactobacillus alimentarius (15.19 %), Lactobacillus plantarum (22.78 %), Lactobacillus fermentum (18.99 %), Lactobacillus brevis (06.33 %), Lactococcus lactis (12.66 %), Lactococcus cremoris (06.33 %), Leuconostoc mesenteroides (06.33 %) and Enterococcus faecalis(11.39 %). was found similarities with traditional Butter, according to Guessas et al., (2012). We have divided the Lactobacilli group into three subgroups as follows: Lactobacillus plantarum homofermentative growing at low temperatures, Lactobacillus Fermentum which is heterofermentative usually growing at high temperatures and unable to grow at low temperatures, Lactobacillus brevis heterofermentative, growing at low temperatures (Orla-jensen, 1919) and Lactobacillus alimentarius a meat species that produces acetoin and homofermentative ( Carr et a.l, 2002). Three isolates were not able to grow in the same conditions but, were able to growth at 10 °C as described by Axelsson (2004). Lactococcus ssp cocci occurring in pairs or short chains, facultative anaerobes and tolerant to a wide range of conditions: temperature (10 – 45°C), pH (4.5 – 10.0) and high sodium chloride concentrations, they tentatively referred to Enterococcus ssp (Gomes, 2010). He rest of selected isolates were cocci, occurring in pairs and chains, were CO2 positive, action positive and ADH negative they tentatively referred to Leuconostoc mesenteroides (Carr et al., 2002).
Table 4: Morphological, cultural, physiological and biochemical characteristics of LAB strains isolated from traditional Butter.
| Strains isolated | ||||||||
| Characteristics | G 1 | G 2 | G 3 | G 4 | G 5 | G 6 | G 7 | G 8 |
| Number of isolates
Gram Catalase Motility Gas from glucose Hydrolysis of: · ADH · Citrate Production of: · Acetoin · Dextrane Growth at different temperature (°C): · 10 · 15 · 37 · 40 · 45 Growth at different pH: · 4 · 6.5 · 9.6 Growth in the presence of NaCl %: · 2 · 4 · 6.5 · 10 · 12 |
12
+ – – –
– V
V –
– + – – –
+ + –
+ DR – – – |
18
+ – – +
– V
– –
– + + – –
+ + –
+ – DR – – |
15
+ – – –
+ V
V –
– – DR + +
– + –
+ – DR – – |
05
+ – – +
+ V
– –
– + + – –
+ + –
+ + – – – |
10
+ – – –
+ V
V –
+ + – DR –
+ + –
+ + – – – |
05
+ – – –
– V
– –
+ + – – –
+ + –
+ – – – – |
05
+ – – +
– V
– +
– + – – –
– + –
V V – – – |
09
+ – – –
– V
+ –
+ DR + + +
– + +
+ + + + DR |
(+): Positive reaction, (-): Negative reaction, DR: delayed reaction. Group 1: 6, 7, 21, 30, 10, 43, 44, 52, 16, 34, 47 and 17. Group 2: 42, 77, 46, 11, 45, 27, 35, 73, 74, 36, 28, 76, 29, 53, 54, 67, 69, and 70. Group 3: 40, 22, 8, 63, 13, 64, 15, 31, 68, 37, 33, 55, 56, 57 and 66. Group 4: 41, 59, 23, 26 and 50. Group 5: 1, 39, 20, 62, 2, 12, 24, 58, 38 and 32. Group 6: 49, 61, 19, 3 and 51. Group 7: 71, 79, 72, 75 and 78. Group 8: 60, 48, 18, 4, 5, 9, 14, 25 and 65.
 |
Table 5: Sugars fermentation of LAB isolated from traditional Butter.
|
Antibacterial Activity
The antimicrobial activity of lactic acid bacteria (LAB) isolated from traditional Butter were detected using the method of well diffusion test on the basis of their ability to inhibit the growth of the indicators isolates Bacillus subtilis ATCC 93 72, Bacillus cereus ATCC 10876, Staphylococcus aureus ATCC 65 38 and Escherichia coli ATCC, L 25 922). Based on the results, the strains of the group 2, group 5 and group 8 showed the largest zone of growth inhibition was selected for further strain developmental studies (Table 6). With regard to the results of other indicator bacteria, Bacillus subtilis ATCC 93 and Escherichia coli ATCC, L 25 922.Were found reflect their ability to inhibition. Inhibitor compounds produced by strains inhibitors showed different of sensitivity. The strains (27, 67, 62, 20, 48, 14 and 65) were completely inactivated by α-chymotrypsin alone which was resistant to pepsin (43, 17, 60, 40 and 50), whereas the compounds produced by 11, 28, 25, and 58 isolates were inactivated after treatment with the lipase, indicating that these substances can have inhibitory lipid moiety in their chemical composition. These results suggest that the biochemical nature of the molecule produced is peptidic. The inhibitory compounds produced by the isolates showed great resilience to thermal treatments. In another way, bacteriocin has proved stable over a wide pH range with all peptides, now some antimicrobial activity in the pH range from pH 4-7. According to Allouche et al (2010). Recent bacteriocin is very sensitive to pH its stability was detected at a pH range of 3.5 to 6.5. In this study, bacteriocin produced by isolates had the same profile and were active at pH values 4- 6. In a similar study, the work of Zamfir et al (1999) Reported that the bacteriocin produced by Lactobacillus acidophilus develop a positive activity against Staphylococcus aureus.
Table 6: Antimicrobial activities of predominant LAB against selected pathogenic bacteria’s.
| Strains percentage.
Agar well-diffusion method (mm) according to selected pathogenic bacteria’s |
|||||
| Strains
group |
Diameter of inhibitory zone |
A |
B |
C |
D |
| Lactobacillus alimentarius (15.19 %)
|
Strong
Intermediate Weak No growth |
22.12 %
18.56 % 10.02 % 49.30 % |
12.12 %
10.06 % 08.02 % 69.80 % |
02.12 %
10.50 % 10.08 % 79.30 % |
01.10 %
04.50 % 07.00 % 87.40 % |
| Lactobacillus plantarum (22.78 %) | Strong
Intermediate Weak No growth |
30.33 %
14.66 % 20.44 % 05.71 % |
33.33 %
24.33 % 22.00 % 20.34 % |
42.33 %
14.66 % 30.66 % 12.35 % |
50.00 %
11.66 % 33.66 % 04.68 % |
| Lactobacillusfermentum (18.99 %) | Strong
Intermediate Weak No growth |
05.33 %
07.66 % 10.66 % 81.30 % |
10.33 %
16.66 % 19.33 % 53.68 % |
14.33 %
14.66 % 20.33 % 50.68 % |
22.33 %
14.66 % 02.66 % 60.35 % |
| Lactobacillus brevis (06.33 %) | Strong
Intermediate Weak No growth |
00.00 %
00.00 % 00.00 % 100.0 % |
00.00 %
00.00 % 00.00 % 100.0 % |
00.00 %
00.00 % 00.00 % 100.0 % |
00.00 %
00.00 % 00.00 % 100.0 % |
| Lactococcus lactis (12.66 %) | Strong
Intermediate Weak No growth |
09.33 %
05.66 % 14.66 % 70.35 % |
17.33 %
11.66 % 16.66 % 54.35 % |
09.66 %
17.66 % 22.66 % 50.02 % |
15.45 %
09.66 % 22.28 % 52.61 % |
| Lactococcus cremoris (06.33 %) | Strong
Intermediate Weak No growth |
00.00 %
00.00 % 00.00 % 100.0 % |
00.00 %
00.00 % 00.00 % 100.0 % |
00.00 %
00.00 % 00.00 % 100.0 % |
00.00 %
00.00 % 00.00 % 100.0 % |
| Leuconostoc mesenteroides (06.33 %) | Strong
Intermediate Weak No growth |
19.53 %
25.66 % 18.68 % 36.13 % |
22.33 %
07.67 % 04.06 % 65.94 % |
11.38 %
11.69 % 12.61 % 64.32 % |
09.33 %
05.66 % 14.66 % 70.35 % |
| Enterococcusfaecalis(11.39 %) | Strong
Intermediate Weak No growth |
02.37 %
05.02 % 10.22 % 77.31 % |
11.03 %
07.68 % 12.44 % 68.85 % |
08.33 %
02.06 % 02.86 % 70.93 % |
22.35 %
11.06 % 22.33 % 44.24 % |
Weak (3-6), Intermediate (7-11), Strong (12-15), No growth. A: Bacillus subtilis ATCC 93 72, B: Bacillus cereus ATCC 10876, C: Staphylococcus aureus ATCC 65 38, D: Escherichia coli ATCC, L 25 922.
The appearance of the zones of the proteolytic activity in the concentrations 1 and 2 % is very easily detected. While in concentration 3 % detection is very low and fully absent in high concentration. In this case, all the strains selected gave a zone of lyses on milieu MRS skimmed milk. Thus, they have a strong proteolytic activity. One chooses the adequate concentration lower than 2 %, to obtain strains with a great proteolytic power. We have chosen to strongly proteolytic strains to the metering casein. From which it can be said that the amount of casein decreases rapidly with time in strains strongly proteolytic with a mean velocity of consumption equalizes with (688 µg/h). These similar results with the results obtained by Atanasovaa et al (2014) and Guetouache et al (2015) for strains Lactobacillus lactis and Lactobacillus plantarum.
Acknowledgements
The authors would like to acknowledge Mohamed Bouadiaf University of M’sila for their willingness and providing laboratory facility. Corresponding Author: Special thanks to my wife for critical review of the manuscript and invaluable help.
Conclusions
The study was conducted to isolate and identify the naturally occurring lactic acid bacteria from traditional butter. With this 97 lactic acid bacteria belonging to the genus Lactobacillus, Lactococcus, Leuconostoc and Enterococcus were identified. The results obtained from the present study demonstrated that there is a diversity of lactic acid bacteria in traditional butter. These organisms are able to produce antimicrobial com-pounds against competing microbiota, including food-borne spoilage and pathogenic bacteria. They were considered as potential candidate lactic acid bacteria for use as starter culture in dairy milk fermented production.
References
- Mechai A., Kirane D. Antimicrobial activity of autochthonous lactic acid bacteria isolated from Algerian traditional fermented milk (Raib). J. Biotech, 2008; 16:2908-2914.
- Rhiat M., Labioui H., Driouich A., Mennane Z., Ouhssine M. Preparation of the starter trial production of chesses (Jben) and (Klila) at laboratory scale. Food Sci. Qua. Manage, 2013; 13:2225-0557.
- Guetouache M., Guessas B. Characterization and identification of lactic acid bacteria isolated from traditional cheese (Klila) prepared from cow’s milk. African Journal of Microbiology Research, 2015; 9(2): 71-77.
CrossRef - Hadj Aissa M. Pour votre culture générale : les produits laitiers fabriqués en Algérie- posté par D.SOUKEHAL, 2011 ; . http://www.sidielhadjaissa.com/article.
- Halasz A. Lactic Acid Bacteria. In: Lasztity R, Food Quality and Standards (Vol. 3), 2009; EOLSS Publishers Co Ltd, UK.
- Carr F.J., Hill D., Maida N. The lactic acid bacteria: A literature survey. Rev. Microbiol, 2002; 28: 281-370.
- Parada J.L., Caron C.R., Medeiros A.B.P., Soccol C.R. Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Brazilian Archives of Biology and Technology, 2007; 50(3): 512-542.
CrossRef - Paneri FP., Christaki E., Bonos E. Lactic acid bacteria as source of functional ingredients. INTECH Open Access Publisher.World’s largest Science, Technology & Medicine, 2013; Open Access book publisher. Publish, read & share novel research.
- National Agency of Investment Development: Interview with DJELLAOUI Abd-el- Kader, Wali of Djelfa, Interview realized by ANDI with the Wali of Djelfa March, 2014; 1-7.
- Meshref A. Microbiological quality and safety of cooking butter in Beni-Suef governorate-Egypt. African Health Sciences, 2010; 10(2): 193–198.
- Guessas B., Fatma A., Miloud H, Mebrouk K. Isolation and Identification of Lactic Acid Bacteria from Dhan, a Traditional Butter and Their Major Technological Traits. World Applied Sciences Journal, 2012; 17 (4): 480-488.
- Ashraf R., Smith S.C. Selective enumeration of dairy based strains of probiotic and lactic acid bacteria. International Food Research Journal, 2015; 22 (6): 2576-2586.
- Khedid K., Faid M., Mokhtari A., Soulaymani A., Zinedine A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiological research, 2009; 164 :81-91.
CrossRef - Mathara J.M., Schillinger U., Kutima P.M., Mbugua S.K., Holzapfel, W.H. Isolation, identification and characterization of the dominant microorganisms of kulenaoto: The Maasai traditional fermented milk in Kenya. J. Food Microbiol, 2004; 94: 267-278.
CrossRef - Neti Y., Erlinda I. D. Phenotypic identification of lactic acid bacteria isolated from Tempoyak (fermented durian) made in the Philippines. J. Biol, 2011; 3: 10-5539.
- Schillinger U., Lucke F.K Antibacterial activity of Lactobacillus sake isolated from meat. Envi Micro, 1989; 55: 1901-1906.
- Accolas J.P., Bloquel R., Didienne R., Regnier,J. Propriétés acidifiantes des bactéries lactiques thermophiles en relation avec la fabrication du yoghourt. Le Lait , 1977 ; 57 : 1-23.
CrossRef - Marroki A., Zúñiga M., Kihal M., Pérez-Martínez G. Characterization of Lactobacillusfrom Algerian Goat’S Milk Based on Phenotypic, 16S rDNA Sequencing and their Technological Properties. Brazilian Journal of Microbiology, 2011; 42(1): 158–171.
CrossRef - Jini R., Swapna H.C, Amit K. R., Vrinda R., Halami P.M, Sachindra N.M., Bhaskar N. Isolation and characterization of potential lactic acid bacteria (LAB) from freshwater fish processing wastes for application in fermentative utilisation of fish processing waste. Braz J Microbiol, 2011; 42: 1516-1525.
CrossRef - Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Biochemistry, 1976; 72: 248-254.
- Akabanda F., Owusu-Kwarteng J., Tano-Debrah K., Parkouda C., Jespersen L. The Use of Lactic Acid Bacteria Starter Culture in the Production of Nunu, a Spontaneously Fermented Milk Product in Ghana. International Journal of Food Science, 2014; 721067.
- Keyvani M. B. Physicochemical and organoleptic properties of Lighvan cheese fortified with Protulaca Oleracea seed oil. Journal of Chemical Health Risk, 2015; In Press.
- Terzic V.A., Mihajlovic S., Uzelag G., Golic N., Fira D., Kojic M., Topisirovic L. J. Identification and characterization of lactic acid bacteria isolated from artisanal white brined Golija cow’s milk cheeses. Biol. Sci. Belgrade, 2014; 66: 10.2298.
- EC (European Commission). Overview of Microbiological Criteria for Foodstuffs in Community Legislation in Force, 2001.
- Orla-jensen. The lactic acid bacteria Copenhagen. Fred. Host-Sen, 1919.
- Axelsson L. Lactic Acid Bacteria, Microbiological and Functional Aspects: Classification and Physiology. Edited by Salminen, S., von Wright A., Ouwehand, A.C. Third Edition. Marcel Dekker Inc, New York: 2004; pp, 634.
- Gomes B.C., de Melo F.B.D.G., De Martinis E.C.P. Dualistic aspects of Enterococcus spp in foods. Technology and education Topics in Applied Microbiology and Microbial Biotechnology, 2010; 1119-1125.
- Allouche F. N., Hellal A., Laraba A. Etude de l’activité antimicrobienne des souches de Lactobacilles thermophiles utilisées dans l’industrie laitière. Nat. Technol, 2010 ; 3 : 13-20.
- Zamfir M., Cailewaert R., Cornea P.C., Savu L., Vatafu L., Devuyst L. Purification and characterisation of a bacteriocin produced by Lactobacillus acidophilus IBB 801. Appl. Microbiol, 1999; 87: 923-931.
CrossRef - Atanasovaa J., Monchevab P., Ivanovab I. Proteolytic and antimicrobial activity of lactic acid bacteria grown in goat milk. Biotechnology & Biotechnological Equipment, 2014; 28 (6): 1073-1078.
CrossRef - Guetouache M., Guessas B., Medjekal S., Toumatia O. Technological and Biochemical characterization of Lactic Acid Bacteria isolated from Algerian Traditional Dairy Products. World Applied Sciences Journal, 2015; 33(2): 234-241.

This work is licensed under a Creative Commons Attribution 4.0 International License.





