Manuscript accepted on : 01 May 2018
Published online on: 08-06-2018
Adawiya J. Haider1, Duha S. Ahmed1 , Azhar J. Bohan2 and Saja M. Jabar2
, Azhar J. Bohan2 and Saja M. Jabar2
1Applied Physics Branch, Applied Sciences Department, University of Technology Iraq.
2Nanotechnology Advanced Material Research Center, University of Technology, Iraq.
Corresponding Author E-mail: duhasaadi2015@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2633
ABSTRACT: New and economic method of functionalized Multi walled Carbon Nanotubes (MWCNTs) was made using neem and flax (kitan) oil separately improving their solubility as compared with the traditional methods. This method is initiated by ultrasoniction mixture of each oil separately (neem and flax) with 0.05g MWCNTs. Then the samples were dried at 90°C in a vacuum oven for 24 h and annealing for 1h at temperature 200oC to obtain the powder of MWCNTs containing functional groups treated with both oil separately. The results were examined by using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) to study the morphology of the surface treated with neem and flax oil. Fourier transformed infrared spectroscopy (FTIR) shows the formation of functional group on to MWCNTs surface such as C=O and COOH. Furthermore, improving the antimicrobial activity of functionalized MWCNTs treated with oils and reducing colonies at high consecrations of samples against E. coli, P. aeruginosa and S. aureus after incubated for 24h which reveals the ability of functionalized MWNTs in removal pathogens and enhanced the adsorption of bacteria on the surface of (MWCNTs), which are used in environmental applications and contaminated water such as filters.
KEYWORDS: and Flax Oil; Bacterial Adsorption; Fatty Acids (Omega-3); Functionalization Mwcnts; Neem Oil; Surfactants
Download this article as:| Copy the following to cite this article: Haider A. J, Ahmed D. S, Bohan A. J, Jabar S. M. Promoting Functionalized Multi Walled Carbon Nano Tubes Using neem and flax oil for Resentence Pathogenic Bacteria. Biosci Biotech Res Asia 2018;15(2). |
| Copy the following to cite this URL: Haider A. J, Ahmed D. S, Bohan A. J, Jabar S. M. Promoting Functionalized Multi Walled Carbon Nano Tubes Using neem and flax oil for Resentence Pathogenic Bacteria. Biosci Biotech Res Asia 2018;15(2). Available from: https://www.biotech-asia.org/?p=30029 |
Introduction
In spite of the great development in human health, the antibacterial activity of plants has been known for several years, such as a plant is recognized to form useful antibacterial phytochemicals.1 Besides, these plants and their active ingredients are used in traditional medicine because of the easy availability, low cost and negligible side effects.2 Neem oil, known as Azadirachta indica is a plant used as a source of therapeutic agents and grows well in the hot countries. It is considered as an important resource of exceptional ordinary product for developing medicines and has antibacterial properties that are used for reducing bacterial contamination.3 Moreover, neem oil compositions contain fatty acids (Omega-3) represented by long chain of linoleic acid, oleic acid and carboxylic acid. Another commercially and traditional oil component isolated from plants is flax (kitan) oil known as Linum usitatissimum which is a species of the family Linaceae and normal plants that developed broadly in Mediterranean. It’s used against a lot of diseases, like skin, respiratory zone and gastrointestinal tract dieses.4-6 The compositions of flax oil is similar to neem oil includes Fatty acids (Omega-3) such as Oleic acid, Linoleic acid and Linolenic acid. In previous works, using L. usitatissimum oil showed a significant anti-inflammatory,7 anti-arthritic8 and anti-diabetic.9 Besides, these oils represented a very big market of minimally routes medicinal plant pieces particularly in Europe and America, which are generally dispensed as over-the-counter medicine. Carbon nanotubes (CNTs) that are represented by Single Walled carbon nanotubes (SWCNT) and Multi walled carbon nanotubes (MWCNT) become a suitable for several applications, with exceptional features like high aspect ratio, mechanical, electrical and thermal properties. CNTs display extraordinary adsorption properties towards different organic and inorganic compounds. The possible of surface functionalization produce attractive for Water treatment.10,11 Recently, searching of biological and medical applications of CNTs has become important field of studies.12 Bacteria and viruses can also be removed effectively by membrane filters made from SWCNTs or MWCNTs.13-18 Performances of CNTs are limited because of their agglomeration and poor dispersion, which result from the van der Waals bonds among the graphene sheets. This drawback can be overcome by surface functionalization. Different methods for functionalization of carbon nanotube have reported in the literature such as non covalent functionalization and covalent functionalization to improve their dispersion ability. The common and simplest method is covalent functionalization under the oxidized treatment by various reagents (e.g.,nitric acid, sulfuric acid , ammonium hydroxide and hydrogen peroxide) producing functional groups such as carboxylic, carbonyl, and hydroxyl on the surface of the CNTs.19-21 The researchers are still working to find a suitable method to modify and functionalize CNTs using expensive method to improve dispersion as reported the side wall functionalization of MWCNTs by organic solvent like olive oil,22 which improved the functionalization MWCNTs by ultrasonic in organic olive oil as solvent.
In this work we develop new and economic method by using plants oil solvent to functionalize MWCNTs like neem and flax oil using ultrasonication for 1h min and increase their dispersion and introduce functional groups which lead to react with compounds and other biological active molecules of various sizes. The results were examined by using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) to study the morphology of the surface treated with neem and flax oil. Fourier transformed infrared spectroscopy (FTIR) shows the formation of functional group on MWCNTs surface such as C=O and COOH.
Materials and Methods
Functionalization of MWCNTs with neem and flax oil
In this step raw-MWCNTs are chemically functionalized by ultrasonication for 1h in separated flask of 100 ml and each one contain neem oil and flax oil, separately to produce carboxyl and hydroxyl functional groups. Firstly, using 0.05g of raw-MWCNTs that treated with 2.5 ml of neem oil in flask of 100ml and the other 0.05g treated with the same quantity flax oil in flask of 100ml. Both flasks were vortex for 10 min and ultrasonic in water bath ultrasonic at temperature of 25°C for 1has shown in Fig (1). Then treated-MWCNTs were filtered from neem oil and flax oil, separately via filter method under vacuum in a Buchner filter with a 0.22 micro- membrane and washed thoroughly 400ml of Chloroform (ChCl3) to remove oil from each sample. The samples were dried at 90°C in a vacuum oven for 24 h and annealing for 2h at temperature 350oC in order to obtain the powder of treated MWCNTs.
 |
Figure 1: Images of treated MWCNTs with neem and flax oil in bath ultrasonic at temperature of 25°C for 1h to obtain carboxyl functional groups.
|
Characteristics of Functionalized MWCNTs
Analysis of chemical compositions of MWCNTs before and after treatment with neem and flax oil are characterized improved by using Fourier Transform Infrared Spectroscopy (FTIR, 8400S, Shimadzu). Besides, microstructure and surface morphology of MWCNTs before and after treated with neem and flax oil are carried out by Transmission Electron Microscope (TEM, Philips EM208, Iran) and Scanning Electron Microscopy (SEM, the VEGA EasyProbe).
Antimicrobial Activities of MWCNTs
Antibacterial activity of the treated-MWCNTs with neem and flax oil, were performed by using plate count method against two types of bacterial strains like E. coli and P. aeruginosa as gram negative and S. aureus as gram positive bacteria supplied by (Biotechnology lab in Center of Nanotechnology and Advanced Material, University of Technology, Iraq). The bacteria are cultured on N. Agar and incubated for 24 h at 37°C. Bacterial suspensions are diluted in saline to obtain bacterial samples with concentration ~107 CFU/ml by 0.5 McFarland standards. One milliliter of bacterial solution was added to samples 1 mg/ml and 3 mg/ml of treated-MWCNTs and incubated in shaker incubator at 37 oC and 165 rpm for 42h. Then, the mixtures were serially diluted in normal saline for 103 and cultured on Molar Hinton Agar (100µl spread out on MHA). The survival colonies in plates are counting after 24 h incubation at 37oC using viable count method.
Results and Discussion
FTIR Analysis
FTIR spectrum is used to determine functional groups of MWCNTs after functionalization with neem and flax oils, respectively and recognize the atomic arrangement and concentrations of chemical bonds present in the sample as illustrated in Fig (2 a, b, c).
FTIR analysis show IR spectrum of raw-MWCNTs in Fig (2a) and the carboxylic groups of treated MWCNTs by neem oil with stretch band of carboxyl groups at 1762.94 cm-1 as illustrated in Fig (2b). Characteristic peaks of hydroxyl group (-OH) range of 3288 to 3705 cm-1, which are attributed to introduction the hydroxyl groups (attributed to OH stretching).The appearance bands at 2981 cm-1 and 2850.79 cm-1 are resulted from asymmetric and symmetric stretch of CH stretching. Peaks at 1641 and 1691.57 cm−1 are resulted from C=C stretching of CNTs.13 Peak of 1575.84 cm-1 were related to the characteristic absorption peak of carbon nanotubes, which proved that MWCNTs were preserved after undergone treatment with oil plant like neem.
In case of treated MWCNTs with flax (kitan) oil, there is absorption peaks signal which are observed in the IR spectrum of functionalized MWCNTs as shown in Fig (2c) indicating the introduction of hydroxyl groups bound successfully as compared with raw-MWCNTs (2a). The peaks at 1591 and 1051 are attributed to the C-O backbone stretching mode. While the peaks at 1670, 1764 and 3217 ~ 3784 cm-1 corresponding to C=C, C=O and O-H bonds, respectively that observed after the samples are treated with flax oil contain fatty acids. Besides, peaks at 2918 and 2970 cm-1 were arising from asymmetric and symmetric stretching of C-H. The peaks at 1442, 1352cm−1 are due to O-H bending deformation in -COOH.
 |
Figure 2: FTIR analysis of a) raw, b) treated MWCNTs with neem oil and c) treated MWCNTs with flax oil.
|
As shown in FTIR results in Fig (2), they imply the efficient of plants oils in functionalized MWCNTs because of the fatty acids in their composition which represented by long chain of O–H and –COOH as agreement with previous results using olive oil in treated MWCNTs.22
TEM Analysis of Functionalized MWCNTs using neem and flax (kitan) oil
Transmission Electron Microscope (TEM), analysis was used to study the morphology of raw-MWCNTs (3 a) and treated MWCNTs with neem and flax oil respectively, at as shown in Fig (3 b,c), respectively. In Fig (3 b), treated MWCNTs with neem oil resulting in short and individual bundles, and there was no MWCNTs structural damaged occurred after treated with neem oil. Besides, TEM micrograph of treated MWCNTs samples with flax (kitan) oil revealed that there were no MWCNTs structural damaged occurred and the twisted tubes of F-MWCNTs results as compared with raw-MWCNTs due to the role of oils, while the length of F-MWNT has no significant change as shown in Fig (3c).
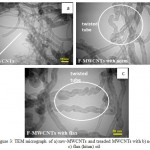 |
Figure 3: TEM micrograph of a) raw-MWCNTs and treaded MWCNTs with b) neem oil and c) flax (kitan) oil.
|
Antimicrobial Activity of Functionalized MWCNTs with neem and flax oils Against Bacteria
As shown in Figs (4) and (5), the antimicrobial activity of treated MWCNTs with neem and flax (kitan) oils are determined using plate counting method. In Fig (4 d,e,f), a number of viable colonies were decreased as the concentrations of treated MWCNTs with neem increased from 1mg/ml to 3mg/ml and become without colonies against three types of bacteria as compared to the Fig (4 a,b,c) using 1mg/ml . Under the same conditions, a number of viable colonies were decreased, exception against gram positive bacteria (S. aureus) as shown in (5 d,e,f) which indicate that treaded MWCNTs with flax oil is less antibacterial agents against S. aureus as compared with MWCNTs treated by neem oil under the same conditions. Moreover, the interaction between the bacteria cell wall and hydrophilic MWCNTs results from cell wall rupture and loss of the integrity of the cell membrane. The percentage of inhibition bacteria reach (99.9%, 99.8% and 31%) for E.coli, Pseudomonas spp. and S. aureus bacteria, respectively at low concentration (1mg) of MWCNTs treated with neem and reach (100% ) at high concentration (3 mg). Besides, the percentage of inhibition bacteria using MWCNTs treated with flax oil reach (98.9% and 100%) for E.coli and Pseudomonas spp at low concentration (1mg) of MWCNTs and reach (100%) for E.coli and Pseudomonas spp bacteria at high concentration of material. While the percentage of inhibition bacteria against S. aureus becomes ( 0% ) in 1 mg and (20% ) in 3 mg when we used MWCNTs treated with flax oil as shown in Fig (6). Since, the results reveals the activity of oils in treated method and absence of Lipopolysaccharide layer in gram positive bacteria that may function as an efficient barrier against any incoming biomolecule.23,24 Besides, another possibility that necessary oils can effectively reduce microbial respiration and increase plasma membrane permeability, that results in loss of bacterial cells after massive ion leakage.25,26
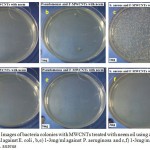 |
Figure 4: Images of bacteria colonies with MWCNTs treated with neem oil using a,d) 1-3mg/ml against E. coli , b,e) 1-3mg/ml against P. aeruginosa and c,f) 1-3mg/ml against S. aureus.
|
There are few studies about the mechanism of antimicrobial activity MWCNTs treated with neem and flax oil that contain Phenolic compound. It suggests that these compounds are multiple cellular targets rather than one particular site of action. Phenolics have exceptional ability to carry out redox interaction of plasma cell membrane and appropriate electrons from the respiration processes. The partial hydrophobicity of phenolics enables them to connect the outer membrane of bacteria and change the wall membrane fluidity. The wall membrane has been penetrated with smaller phenolic compounds that entering the cell membrane and disrupt metabolism.27-29
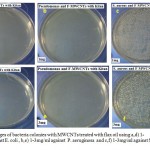 |
Figure 5: images of bacteria colonies with MWCNTs treated with flax oil using a,d) 1-3mg/ml against E. coli , b,e) 1-3mg/ml against P. aeruginosa and c,f) 1-3mg/ml against S. aureus.
|
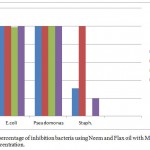 |
Figure 6: The percentage of inhibition bacteria using Neem and Flax oil with MWCNTs in (1 ,3 mg/ml) concentration.
|
Scanning Electron Microscopy (SEM) micrographs of bacteria adhesion
SEM micrographs in Figs (7 a,b,c) and (8 a,b,c), indicate antibacterial activity of functionalized MWCNTs with neem and flax (kitan) oil. Besides, the results indicate the interaction between treated MWCNTs and pathogen like (E. coli, P. aeruginosa and S. aureus) with direct oxidation and morphological changes of E. coli, P. aeruginosa and S. aureus adsorbed on treated MWCNTs deposited on PVDF filter, respectively.
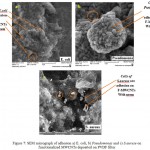 |
Figure 7: SEM micrograph of adhesion a) E. coli, b) Pseudomonas and c) S.auruse on functionalized MWCNTs deposited on PVDF filter.
|
Moreover, it is remarkable that some of treated MWCNTs bundle wounded around curved surfaces of the E. coli, P. aeruginosa and S. aureus due to flexibility of the bundles after Functionalization process which appears to be smaller than the bacterial cell membrane that leads to penetrate the cell wall membrane, changing of the permeability of the membranes and losing the integrity of cell induced by treated MWCNTs.
 |
Figure 8: SEM micrograph of adhesion a) E. coli, b) Pseudomonas and c) S.auruse on functionalized MWCNTs deposited on PVDF filter.
|
Conclusion
New and economic method by using plants oil solvent like neem and flax (kitan) oil to functionalize MWCNTs were improved as shown in FTIR and TEM results which revels the efficient of plants oils in functionalized MWCNTs because of the fatty acids in their composition which represented by long chain of O–H and –COOH and there is not extreme fragmentation of MWCNTs papered after oil treatments. Furthermore, improving the antimicrobial activity of MWCNTs treated with oils and resulted in removal pathogens and reduced number of colonies at high consecrations of treated MWCNTs against E. coli, P. aeruginosa and S. aureus.
Acknowledgements
Authors gratefully acknowledge the Center of Nanotechnology and Advanced Material, University of Technology, Baghdad, Iraq for helping and performing SEM (VEGA Easy Probe), (FTIR,8400S, Shimadzu model) spectra, TEM analysis Day Petronic Co., (Tehran, Iran) and Biotechnology lab in center for performing special antibacterial activity tests.
Conflict of Interest
There is no Conflict of Interest
References
- Cowan M M. Plant products as antimicrobial agents. Clinical Microbiology Reviews. 1999;12(4):564-582.
- Mohammad A. Antimicrobial Potential of Azadirachta indica Against Pathogenic Bacteria and Fungi. Journal of Pharmacognosy and Phytochemistry. 2012;1(4).
- Abhishek M, Banasthali V. Antibacterial Effects of Crude Extract of Azadirachta Indica against Escherichia Coli And Staphylococcus Aureus International Journal of Science. Environment and Technology. 2013;2(5):989–993.
- Prasad K. Secoizolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab Clin Med. 2001;138(1):32–39.
CrossRef - Christofidou-Solomidou M, Tyagi S, Tan K S, Hagan S. S, Pietrofesa R, Dukes F, Arguiri E, Heitjan D F. et al, Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer. 2011;11:269.
CrossRef - Miśta D, Kroliczewska B, Zawadzki W, Pecka E, Steininger M. et al, The effect of Linola and W92/72 transgenic flax seeds on the rabbit caecal fermentation–in vitro study. Pol .J. Vet Sci. 2011;14(4):557–564.
CrossRef - Kaithwas G, Mukherjee A, Chaurasia A. K, Majumdar D. K. Antiinflammatory, analgesic and antipyretic activities of L. usitatissimum (flaxseed/linseed) fixed oil. Indian Journal of Experimental Biology. 2011;49(12):932–938.
- Kaithwas G, Majumdar D. K. Therapeutic effect of Linum usitatissimum (flaxseed/linseed) fixed oil on acute and chronic arthritic models in albino rats. Inflammopharmacology. 2010;18(3):127–136.
CrossRef - Kaithwas G, Majumdar D. K. In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. European Journal of Lipid Science and Technology. 2012;144(11):1237–1245.
CrossRef - Ntim S. A, Mitra S. Removal of Trace Arsenic to Meet Drinking Water Standards Using Iron Oxide Coated Multiwall Carbon Nanotubes. Chem Eng Data. 2011;56(5):2077–2083. doi:10.1021/je1010664.
CrossRef - Park J. E, Kim G. R, Yoon D. J, Sin C.H, Park I.S, Bea T.S, et al. The Effect of Dispersed MWCNTs Using SDBS Surfactant on Bacterial Growth. World Academy of Science, Engineering and Technology. 2012;70.
- Eduardo R.H, Gabriel A. N, Camarena P. Juan, Heriberto E.G, Ratnasamy S. Side-Wall Functionalization of Multi-Walled Carbon Nanotubes with t-Butyl Diazoacetate. J. Mex. Chem. Soc. 2011;55(1):7-10.
- Brady-Estevez A.S, Kang S and Elimelech M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small. 2008;4:481–484.
CrossRef - Brady-Estévez A. S, Nguyen T. H, Gutierrez L, Elimelech M. Impact of solution chemistry on viral removal by a single-walled carbon nanotube filter. Water Research. 2010a;44:3773–3780.
CrossRef - Brady-Estevez A.S, Schnoor M.H, Kang S and Elimelech M. SWNT–MWNT hybrid filter attains high viral removal and bacterial inactivation. Langmuir. 2010b;26:19153–19158.
CrossRef - Kang S, Mauter M.S, Elimelech M. Microbial cytotoxicity of carbon-based nanomaterials: implications for river water and wastewater effluent. Environmental Science and Technology. 2009;43(26):48–2653.
CrossRef - Qu X, Alvarez Pedro J, Li Q. Applications of nanotechnology in water and wastewater treatment. water research. 2013;47:3931-3946.
CrossRef - Srivastava A, Srivastava O. N, Talapatra S, Vajtai R and Ajayan P. M. Carbon nanotube filters. Nature Materials. 2004;3(9):610-614.
CrossRef - Avile´F. S, Cauich-Rodr ´Guez J.V, Moo-Tah L. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon. 2009;47:2970 –2975.
CrossRef - Datsyuk V, Kalyva M, Papageli K, Parthenio J, Tasis D. Chemical oxidation of multiwalled carbon nanotubes. Carbon. 2008;46:833-840.
CrossRef - Om K, Yamini S, Rao V.K, Vijayaraghavan R. Carbon Nanotubes: Detection of Chemical and Biological Warfare Agents. Defence Science Journal. 2008;58(5):617-625.
CrossRef - Duha S. Ahmed, Adawiya J. Haider, Mohammad R. Mohammed. Comparesion of Functionalization of multi walled Carbon nanotubes treated by oil olive and nitric acid and their characterization. Energy Procedia. 2013;36:1111-1118.
CrossRef - Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogen by gaseous contact. J. Antimicrob. Chemoth. 2001;47: 565-573.
CrossRef - Delaquis P.J, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002;74:101-109.
CrossRef - Lambert R.J, Skandamis P.N, Coote P.J, Nycas G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001;91:453-462.
CrossRef - Walsh S.E, Maillard J.Y, Russel A.D, Catrenich C.E, Charbonneau D.L, Bartolo R.J. Activity and mechanism of action of selected biocidal agents on Gram – positive and negative bacteria. J. Appl. Microbiol. 2003;94:240-247.
CrossRef - Cushnie P.T, Lamb A.J. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 2005;26:343-356.
CrossRef - Lacombe A, Vivian C .H, Tyler S, Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. International Journal of Food Microbiology. 2010;139:102-107.
CrossRef - Kwon Y .I, Apostolidisa E, Labbea R G, Shettya K. Inhibition of Staphylococcus aureus by phenolic phytochemicals of selected clonal herbs species of Lamiaceae family and likely mode of action through proline oxidation. Food Biotechnology. 2008;21:71-89.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





