Manuscript accepted on : 04 May 2017
Published online on: --
Tawheed Amin, H. R. Naik and Syed Zameer Hussain
Division of Post-Harvest Technology, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir, Shalimar Campus, Srinagar 190 025, Jammu and Kashmir, India.
Corresponding Author E-mail: tawheed.amin@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2537
ABSTRACT: The Aim of the Present Study Was to Evaluate the Yield, Chemical Constituents and Determine the Chemotype of the Essential Oil Obtained from Different Rosemary Plants Growing in Different Accessions of Rosemary Fields. About four Plant Samples Were Analyzed for Essential Oil Yield and the Essential Oil Yield Varied from 0.88% To 1.2%. the Essential Oil Samples Were Further Analyzed by Gas Chromatography (GC) for the Purpose of Identification of Chemical Constituents Present in Them. It Was Contended from the Results That the Selected Plants Differed from Each Other in Terms of Chemical Constituents.Camphor Content Was Foundin Higher Amount in All the Foursamples, Thus it Could Be Inferred That the Plants Are Camphor Chemotype.
KEYWORDS: Rosemary; Essential oil; Camphor; Gas Chromatography; Terpenoids
Download this article as:| Copy the following to cite this article: Amin T, Naik H. R, Hussain S. Z. Chemotypingthe Essential Oil Indifferent Rosemary (Rosmarinus officinalis L.) Plants Grown in Kashmir Valley. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Amin T, Naik H. R, Hussain S. Z. Chemotypingthe Essential Oil Indifferent Rosemary (Rosmarinus officinalis L.) Plants Grown in Kashmir Valley. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27019 |
Introduction
Rosmarinus officinalis L. is a perennial herb with an evergreenneedle-like leaves that belong to the Lamiaceae (mint) family1-3. Rosemary is a widely used aromatic and medicinal plant4,5. The leaf of rosemary is an indispensable spice of the French, Italian and Spanish cuisine. Rosemary is cultivated for the valuable oil, whichcan be extracted from the harvested plants when flowers are in buds5.
Essential oils arenatural, concentrated, hydrophobic liquids containing volatile aroma compounds from plants1. They are also known as volatile or ethereal oils. The volatile or essential oils correspond to a mixture of hemiterpenoids, monoterpenoids and some sesquiterpenoids that are in conjunction with oil. These mixtures are highly volatile when exposed to air at room temperature, thus the name ethereal oils.They are almost insoluble in water and soluble in alcohol and usually lighter than water. They have high refractive index and many of them are optically active. Essential oils are generally extracted by distillation. The chemical composition of an essential is quite different from one plant to another and the main chemical constituents present in essential oil determine its aroma, taste and biological activity.
There is a wide range of techniques which have been used for extraction and concentration of essential oils as well as chromatographic separation and identification of chemical constituents present in essential oils. Extraction of volatile terpenoids from plant materials and from a wide variety of other matrices is often carried out using hydrodistillation or steam distillation6.Rosemary oil was notified for Generally Recognized as Safe (GRAS) status by the Fragrance and Essence Manufacturers Association of the USA (FEMA) in 1965 and has been listed by the U.S. Food and Drug Administration (FDA) for food use (GRAS)2. In 1970, the Council of Europe included rosemary oil in the list of substances, spices and seasonings deemed admissible for use, with a possible limitation of the active principles in the final product2,7.
It is generally known that the components of essential oils from aromatic plants of the same scientific name could be different according to the plant’s habitats, or parts and methods for extraction. This variation in composition is called chemotype8. Chemotype occurs when aromatic plants grow under different climatic and soil conditions8,9.
The present study was therefore, carried out to evaluate the yield, chemical composition and determine the chemotype of different rosemary plants grown in Kashmir valley.
Materials and Methods
Sample Collection
Fresh leaves of RosmarinusofficinalisL.were collected 15minutes prior to distillation of essential oil, from the fields of Indian Institute of Integrative Medicine (IIIM)Sanatnagar, Srinagar, Jammu& Kashmir, India.
Distillation of Essential oil
The essential oil was obtained by hydrodistillation in a Clevenger (PERFIT, INDIA) for 3 hours3,10. Briefly, 250g of freshly collected leaves weretaken in 500 ml round-bottom flask (PERFIT, INDIA)followed by addition of water in the ratio of 1:6 (w/v) and was distilled for about 3 hours.Essential oil from each of the sampleswas collected and dried over anhydrous sodium sulphate3,11. Oil was then stored at 4oC until analysis with Gas chromatography (GC)3.
Chromatography
The analysis of the oil was carried out by following the method of Amin et al.(2013)on a gas chromatograph Perkin Elmer – Auto XL equipped with head space analyzer and FID, using a fused-silica capillary column (30m x 0.32 mm; 0.25 μm film thickness). The oven temperature was programmedfrom 60oC to 250oCat 5oC per minute.The injector and detector temperatures were set at 250oC and 270oC, respectively. Nitrogen at a pressure of 8 psi was used as the carrier gas.The identification was done on the basis of retention time, Kovats index, MS Library search (NIST & WILEY). Retention indices (RI) of the chemical components of samplesand authentic compounds were determined.The relative amounts of the identified compounds were calculated based on GC peak areas without using correction factors.
Results and Discussion
Yield of Essential Oil
The yield of essential oil from each of the foursamples ranged from 0.88% to 1.2% (Figure 1). It is clear from Figure 1that the highest essential oil content was found from plant – P55/B2(1.2%)and lowest yield from plant P57/B2(0.88%).
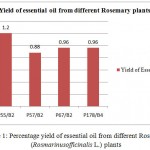 |
Figure 1: Percentage yield of essential oil from different Rosemary (Rosmarinus officinalis L.) plants
|
Identification of Compounds
Gas chromatographic analysis of the essential oil resulted in the identification of 18 different components representing about 97.2217% of the essential oil of P55/B2; 19 components representing 97.6266% of the essential oil of P57/B2; 17 components representing about 76.5109% of the essential oil of P67/B2 and 22 components representing about 86.0686% of the essential oil of P178/B4.All of the identified compounds and their percentagepresent in each of the plant are summarized in Tables 1, 2, 3 & 4. Camphor was found to be present in the highest concentration (53.3871%) which is much higher than that reported by Vermaet al.10followed by 1, 8- cineole (11.6516%), alpha-pinene (5.6862%) and camphene (5.4258%). It is thus clear from the results that all the plants are camphor chemotype.
Table 1: Composition of essential oil of Rosemary (Rosmarinusofficinalis L.) (P55/B2)
| Compound | RT (min) | RI or KI | Peak Area (%) |
| Alpha-pinene | 6.546 | 937.3.0 | 7.2730 |
| Sabinene | 7.284 | 961.8 | 5.3293 |
| Beta pinene | 7.643 | 972.6 | 6.9296 |
| Mycrene | 8.083 | 988.0 | 1.9276 |
| Alpha phellandrene | 8.530 | 1001.0 | 0.2754 |
| Alpha terpinene | 8.844 | 1012.2 | 7.6594 |
| 1,8-cineole | 9.473 | 1032.0 | 13.6826 |
| (E)- beta ocimene | 9.870 | 1042.8 | 1.5131 |
| (Z) – sabinene hydrate | 0.925 | 1069.7 | 0.5493 |
| Camphor | 13.946 | 1146.0 | 38.6226 |
| Borneol | 14.693 | 1165.1 | 5.0088 |
| Terpinen-4-ol | 15.075 | 1173.7 | 0.6782 |
| Alpha terpineol | 15.870 | 1190.5 | 1.6530 |
| Verbenone | 16.601 | 1204.0 | 0.4285 |
| Bornyl acetate | 19.323 | 1287.0 | 4.7110 |
| Beta caryophyllene | 23.830 | 1417.0 | 0.2279 |
| Alpha humulene | 25.217 | 1453.2 | 0.1997 |
| Caryophyllene oxide | 30.832 | 1582.5 | 0.5527 |
| Total identified97.2217 | |||
 |
Figure 2: Gas chromatogram of essential oil Rosmarinusofficinalis L. (P55/B2)
|
Table 2: Composition of essential oil of Rosemary (Rosmarinusofficinalis L.) (P57/B2)
| Compound | RT (min) | RI or KI | Peak Area (%) |
| Alpha-pinene | 6.556 | 936.0 | 5.6862 |
| Camphene | 7.294 | 954 | 5.4258 |
| Beta pinene | 7.713 | 976 | 0.7221 |
| Mycrene | 8.094 | 983.9 | 2.7746 |
| Delta-3-carene | 8.867 | 1007.7 | 5.3275 |
| 1,8-cineole | 9.566 | 1030.2 | 11.6516 |
| (E)- beta ocimene | 9.904 | 1040 | 0.4748 |
| (Z) – sabinene hydrate | 10.927 | 1065.8 | 0.7116 |
| Linalool | 12.108 | 1092.8 | 0.9980 |
| Camphor | 14.064 | 1141 | 53.3871 |
| Borneol | 14.731 | 1165 | 1.6864 |
| Terpinen-4-ol | 15.13 | 1174 | 0.6502 |
| Alpha terpineol | 15.88 | 1186 | 1.33 |
| Verbenone | 16.343 | 1202 | 0.0366 |
| Nerol | 17.529 | 1233.5 | 1.6855 |
| Bornyl acetate | 19.365 | 1287 | 4.2267 |
| Beta caryophyllene | 23.823 | 1417 | 0.6217 |
| Alpha humulene | 25.248 | 1453.5 | 0.0484 |
| Caryophyllene oxide | 30.923 | 1580.7 | 0.1818 |
| Total identified | 97. 6266 |
RT: Retention time; KI: Kovat index (relative to n-alkane)
 |
Figure 3: Gas chromatogram of essential oil Rosmarinusofficinalis L. (P57/B2)
|
Table 3: Composition of essential oil of Rosemary (Rosmarinusofficinalis L.) (P67/B2)
| Compound | RT (min) | RI or KI | Peak Area (%) |
| Alpha-pinene | 6.598 | 936.0 | 3.2764 |
| Sabinene | 7.309 | 967.4 | 3.2513 |
| Beta pinene | 7.663 | 973.6 | 2.4618 |
| Alpha phellandrene | 8.135 | 1001.0 | 1.7835 |
| Alpha terpinene | 8.556 | 1012.7 | 0.3062 |
| 1,8-cineole | 8.874 | 1032.2 | 6.4457 |
| (E)- beta ocimene | 9.482 | 1043.3 | 4.2923 |
| (Z) – sabinene hydrate | 10.971 | 1069.8 | 1.2216 |
| Linalool | 12.102 | 1095.0 | 0.3874 |
| Camphor | 13.959 | 1146.0 | 34.3253 |
| Borneol | 14.662 | 1164.3 | 5.0162 |
| Terpinen-4-ol | 15.066 | 1173.5 | 0.7620 |
| Alpha terpineol | 15.737 | 1188.0 | 3.2681 |
| Nerol | 17.555 | 1277.0 | 1.5419 |
| Bornyl acetate | 19.285 | 1287.0 | 6.7744 |
| Beta caryophyllene | 23.854 | 1417.6 | 0.4346 |
| Caryophyllene oxide | 30.856 | 1582.5 | 0.9622 |
| Total identified | 76.5109 |
RT: Retention time; KI: Kovat index (relative to n-alkane)
 |
Figure 4: Gas chromatogram of essential oil Rosmarinusofficinalis L. (P67/B2)
|
Table 4: Composition of essential oil of Rosemary (Rosmarinusofficinalis L.) (P17/B4)
| Compound | RT (min) | RI or KI | Peak Area (%) |
| Alpha-pinene | 6.574 | 936. | 6.0510 |
| Camphene | 6.822 | 947.0 | 0.1545 |
| Sabinene | 7.299 | 967.0 | 4.7442 |
| Beta pinene | 7.658 | 973.4 | 4.2248 |
| Mycrene | 8.078 | 987.8 | 2.1758 |
| Alpha phellandrene | 8.480 | 1001.0 | 0.5840 |
| Alpha terpinene | 8.857 | 1013.6 | 0.6961 |
| 1,8-cineole | 9.505 | 1032.9 | 11.5797 |
| (E)- beta ocimene | 9.872 | 1042.9 | 1.3948 |
| (Z) – Sabinene hydrate | 10.933 | 1069.9 | 0.8736 |
| Linalool | 12.025 | 1095.0 | 1.5214 |
| Chrysanthenone | 13.314 | 1129.1 | 0.1002 |
| Camphor | 14.004 | 1146.0 | 28.2261 |
| Borneol | 14.719 | 1165.8 | 3.8690 |
| Terpinen-4-ol | 15.012 | 1174.0 | 1.4618 |
| Alpha terpineol | 15.797 | 1188.9 | 2.3279 |
| Verbenone | 16.663 | 1204.0 | 0.1357 |
| Nerol | 17.432 | 1227.6 | 7.4616 |
| Bornyl acetate | 19.323 | 1287.0 | 7.2866 |
| Beta caryophyllene | 23.829 | 1417.0 | 1.0323 |
| Alpha humulene | 25.231 | 1453.6 | 0.1675 |
| Caryophyllene oxide | 30.827 | 1581.9 | 2.9385 |
| Total identified86.0686 | |||
RT: Retention time; KI: Kovat index (relative to n-alkane)
 |
Figure 5: Gas chromatogram of essential oil Rosmarinusofficinalis L. (P17/B4)
|
It is clear from the results that the yield of essential oil from samples of different plants varied considerably. The composition of the essential oil varies from plant to plant as can be seen in the tables 2, 3, 4 and 5. The camphor content of the essential oil for all the 4 samples is higher than the value of 15.64% and 22.01% reported byVermaet al.10 and Shawl12, respectively. The camphor content also varies within the samples. Since the camphor content of essential oil is higher, therefore, all the samples are camphor chemotype. The chemical composition of oil depends on how and where the plant was grown, harvested and distilled. When the conditions cause permanent variation in the chemical composition of essential oil of rosemary plants, such plants are calledchemotypes. Three principal chemotypes of R. officinalis L. have been reported which include camphor/borneol, cineole and verbenon13.Typical components of rosemary are 1,8-cineole, α- pinene and camphor14and the relatively stable ratio of these components defines each chemotype. It is known that the rosemary oils are widely divided into two chemotypes by the ratio of major components; one with more than 40% of 1,8-cineole and the other with almost the same percentage of 1,8-cineole, α-pinene and camphor15. These findings are in concomitance with our present results.
Acknowledgment
Author is highly grateful to Indian Institute of Integrative Medicine, Sanatnagar, Srinagar, Jammu& Kashmirfor providing an opportunity to work in their lab.
Conflict of Interest
The author reports no conflict of interest.
References
- Amin, T., Sharma. N., Bhat, S.V. A general overview on Rosmarinusofficinalis L. (Rosemary) as a medicinal plant.Med. Plant: Int. J. Phytomed. Related Ind., 2012a; 4(3): 177-81.
- Amin, T., Gulleria, S.P., Bhat, S.V. Potential applications of Rosemary (Rosmarinusofficinalis L.) as a natural food additive.Res. J. Agric. Sciences (An Int. J.), 2012b; 3(6): 1165-9.
- Amin, T., Bhat, S.V., Shrma, N. Antimicrobial properties of Rosemary (Rosmarinusofficinalis L.) essential oil against Sacharomycescereviseae. Asian J. Microbiol., Biotech. Environ. Sci., 2013; 15(4): 107-10.
- Joseph, S. Advances in essential oils analysis using comprehensive two-dimensional GC and time-of-flight mass spectrometer (GCxGC-TOFMS) Detection. Life Sci. Chem. Anal. Sol., 2008.
- Éva Stefanovits-Bányai, M. H. Antioxidant effect of various rosemary (Rosmarinusofficinalis L.) clones. Acta Biologica Szegediensis, 2003; 111-13.
- Guenther, E.: Oil of Bay. In The Essential Oils. Van Nostrand: New York, 1966; pp 378-96.
- Opdyke, D.L.J. Monographs on fragrance raw materials. Food Cosmet. Toxicol., 12 supplement, 807
- Stewart, E. The chemistry of essential oils made simple: God’s love manifest in molecules. Care Publications, Marble Hill. 2005.
- Mills, S.Y. The scientific foundation for herbal medicinal products.2nd Edition, European Scientific Cooperative on Phytotherapy, Exeter. 2003.
- Verma, R.S., Sashidhara, K.V., Yadav, A. Essential oil composition of ‘blue flower rosemary’ (Rosmarinusofficinalis L.) from subtropical India. Acta Pharmaceutica Sciencia, 2010; 52: 427-30.
- Salehi, P., Fakhari, A.R., Ebrahimi, S.N., Heydari, R. Rapid essential oil screening of Rosmarinusofficinalis L. by hydrodistillation–headspace solvent microextraction. Flavour Fragrance J., 2007; 22(4): 80-285.
CrossRef - Shawl, A., Nisar, S., Kumar, T., Nawchoo, I.A. Essential oil composition of Rosmarinusofficinalis L. cultivated in Kashmir valley – India. Indian Pertumer., 2008; 47-9.
- Guetat, A., Al-Ghamdi, F.A., Osman, A.K. 1, 8-Cineole, α-Pinene and Verbenonechemotype of essential oil of species Rosmarinusofficinalis L. from Saudi Arabia. Int. J. Herbal Med. 2014; 2(2): 137-41.
- Flamini, G., Cioni, P.L., Morelli, I., Macchia, M. and Ceccarini, L. Main agronomic-productive characteristics of two ecotypes of Rosmarinusofficinalis L. and chemical composition of their essential oils.J. Agric. Food Chem., 2002; 50: 3512-17.
CrossRef - Özcan, M.M., Chalchat, J.C. Chemical composition and antifungal activity of rosemary (Rosmarinusofficinalis L.) oil from Turkey.Int. J. Food Sci. Nutr., 2008; 59: 691-98.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





