Manuscript accepted on : 17 August 2016
Published online on: --
Plagiarism Check: Yes
Arlene Thomas, Niraja Ranadive, Harisha Nayak, Sneha Surendran, Madhavan Nampoothiri, Jayesh Mudgal and Yogendra Nayak
Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal University, Manipal-576104, Karnataka, India.
Corresponding Author E-mail: yogendra.nayak@manipal.edu
DOI : http://dx.doi.org/10.13005/bbra/2310
ABSTRACT: Pathology of cancer involves abnormal cell growth and metastasis of abnormally grown cells to other systems of body different form their origin. These mass of abnormal cell growth are termed as neoplasm or tumour.Alarming prevalence of cancer states that diagnosis of cancer is crucial in the survival of patients. Early diagnosis and adequate treatment helps in controlling the prognosis of disease. The prevalence witnessed majority of males getting affected by prostate cancer around a 43% and women diagnosed with breast cancer around a 41%. By understanding the magnitude getting affected by breast cancer, we performed tests on the breast cancer cell lines to provide with more novel drug treatments for breast cancer. Cytotoxic activity of flavonoids which includes morin and chrysin, and resveratrol, a natural polyphenol was checked individually on MCF7 breast cancer cell lines through MTT assay and the data was analyzed which could lead to new novel breast cancer treatments.
KEYWORDS: Morin; chrysin; resveratrol; MCF-7; breast cancer; cytotoxicity
Download this article as:| Copy the following to cite this article: Thomas A, Ranadive N, Nayak H, Surendran S, Nampoothiri M, Mudgal J, Nayak Y. In Vitro Cytotoxicity Activity of Chrysin, Morin and Resveratrol Against MCF-7 Breast Cancer Cell Lines. Biosci Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Thomas A, Ranadive N, Nayak H, Surendran S, Nampoothiri M, Mudgal J, Nayak Y. In Vitro Cytotoxicity Activity of Chrysin, Morin and Resveratrol Against MCF-7 Breast Cancer Cell Lines. Biosci Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15685 |
Introduction
Cancer refers to an extensive group of related diseases in which a few of the body’s cells start dividing without control and invade to the neighboring tissues. The genesis of cancer can occur in any region of the body. In normal cases, human cells develop and multiply to generate new cells as required by the body. On aging or receiving damage, these cells die and are replaced with new cells. However, disruption of this cell cycle process is the major cause of cancer. As the abnormality of these cells increases, aged or harmed cells survive when they ought to be dying. Furthermore, new cells form when they are not necessary. These additional units might multiply without ceasing and is manifested as growths known as tumors. Malignant tumors are able to diffuse into, or attack neighboring tissues. Furthermore, as these tumors develop, few cells could sever and head out to places far away sites in the body through the blood alternately lymph system and result in new tumors distant from the primary tumor. Contrary to malignant tumors, benign tumors show no invasion of neighboring tissues. Benign tumors may sometimes be quite large in size. Nonetheless on removal, they normally don’t grow back, while malignant tumors grows.1
Breast cancer which originates in the mammillary tissue manifested as a change in breast shape with presence of lump and exudate releasing out from nipple.In case of metastasis, the additional manifestations include lymph node swelling, bone hyperalgesia, yellow skin and shortness of breath. Two important genes i.e. breast cancer (BRCA)-1 and BRCA-2 affect the capacity of cells to undergo homologous recombination and DNA repair. The molecular and genetic analysis have linked the mutation in the BRCA-1 and BRCA-2 genes as one of the cause for breast cancer. BRCA1-related breast tumours are considered as triple negative cancers with high-grade and early onset. These tumours lack expression of oestrogenand progesterone receptors along with Her2 (erBB2) receptors.1
In 1970, the breast cancer cell line was isolated from a 69-year-old Caucasian woman. It was named as MCF-7 (Michigan Cancer Foundation-7), referring to the institute in Detroit where Herbert Soule and co-workers established the cell line in 1973. MCF-7 cells are primary tumor of invasive breast ductal carcinoma type and possess estrogen receptors thus they proliferate in presence of estrogen signalling.2 These cell originates from plural effusioin and also comprises progesterone but no ERBB2 gene amplification. Similar to differentiated mammary epithelium, these cell lines process estradiol via its receptor i.e. estrogen receptor to form domes.
The progression of cancer is found to be dependent on various factors one of which is the activation or upregulation of the transcription factor NFkB.3In normal cells, the activation of NFkB is tightly regulated and is activated only when particular stimuli such as generation of reactive oxygen species are provided; while removal of these stimuli, NFkB returns to the inactive state. However, in cancer cells, NFkB becomes constitutively activated due to the molecular alterations in the cell resulting in the change in the expression levels of its target genes which control apoptosis, cell proliferation, cell cycle control and migration.3Hence, it has been hypothesized that inhibition of NFkB improves the efficacy of cancer treatment. It has also been reported that most polyphenols suppress the expression of NFkB. Polyphenols are also known to counteract free radical generation by activating the production of antioxidant enzymes, increase the levels of intracellular antioxidants and scavenge free radicals.
Combination of plant polyphenols with anti-cancer molecules is a novel treatment strategy for cancer chemotherapy.4, 5As reported, approximately more than fifty drugs have been discovered as a chemotherapeutic agents for the treatment of cancer.6These chemotherapeutic agents elicit various side effects apart from their anticancer potential.7Thus, there is a need to search a better molecule in terms of lower side effect.
Chrysin, a naturally occurring flavonoid, is being tested for its cytotoxity potential against prostate and lung cancer.8, 9Apoptotic mechanism of chrysin has been investigated and reported in various cancer cell lines including breast cancer cells such as MDA-MB.10 MDA-MB cell lines are triple negative (ER, PR and HER2 negative). However, there is a dearth of evidence on the cytotoxicity data of chrysin on MCF cell lines, which are ER and PR positive but HER2 negative. Similarly, resveratrol, which is a stilbenoid present in berries, grapes, jackfruit etc., has been proven to possess anti-cancer, anti-oxidant, cardioprotective, anti-inflammatory, anti-aging, and neuroprotective properties. Present study was aimed to evaluate the dose dependent cytotoxity potential of resveratrol against MCF-7 breast cancer cell lines.
Another naturally occurring flavonoid morin obtained from almond, figs and other Moraceae fruits, possess diverse biological activities namely anti-inflammatory, anti-cancer and anti-oxidant.11 However, the anticancer effect of morin on metastacic cancer cell was notbeen established. Based on above evidences, present study was designed to evaluate these three active principles of natural origin for comparative cytotoxic effect against breast cancer.
Materials and Methods
Procurement and maintenance of cells
MCF-7 cell line was procured from National Centre for Cell Science, Pune, India and were grown in 25 cm2 tissue culture flasks and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37ºC in CO2 incubator in humidified atmosphere of 5% CO2 and 95% air. The cells were maintained by routine sub culturing in 25cm2 tissue culture flasks.
Sub-culturing of cells12, 13
Tissue culture flasks were observed under inverted microscope to find the degree of confluency and assure the absence of microbial contaminants. Medium utilized by cells was discarded and cell monolayer was washed with trypsin-EDTA solution (1ml approx.). To the flasks, 0.5 ml Trypsin-EDTA solution was added such as to cover the monolayer and the flask was kept in incubator for 1-2 min. After incubation, the cell detachment was confirmed by visualizing under the inverted microscope. Once the cell detachment was confirmed, DMEM containing 10% FBS (3 ml) was added and mixed. Cell viability was determined in the suspension by Trypan blue dye exclusion assay. From the stock cell suspension, 1×104 viable cells/ml were seeded in 25 cm2 tissue culture flask containing about 4 ml of fresh media and incubated until the flasks attained 60-70% confluency. This process was repeated as demanded by the growth characteristics of the cell line.
Counting of cells
The cells in the T-25 flask were trypsinized to form a cell suspension.The Neubauer’s chamber was previously cleaned with 70% ethanol and kept ready for use.In an eppendorf’s tube, 20µl each of the cell suspension and trypan blue dye were added and mixed well.The required quantity was loaded on the chamber and observed under an inverted microscope.All the live cells were counted from the four big squares of the chamber.
In vitro cytotoxic assay14, 15
Exponentially growing cells were harvested from T- 25 tissue culture flasks.Cells were seeded (5000 cells/well) in sterile 96-well flat bottom tissue culture plate and allowed to attach for 24h.Test compounds were dissolved in DMSO and serially diluted with DMEM to get the different concentrations of (31.25-500 µM). The final concentration of DMSO was not more than 0.2%.After 24 h of incubation, cells were treated with 100μl of test compounds for 48 h. Cells in the control group received only the medium. Each treatment was performed in triplicates.50μl of MTT reagent (Stock: 2mg/ml in PBS) was added to each well and incubated for 3 h at 37ºC. To each wells, 100μl of 100% DMSO was added to solubilize the formazan crystals and the optical density (OD) was measured by an ELISA-plate reader at 540nm. Percentage viability of each compound was calculated by the formula
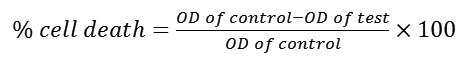
Results and Discussion
Cytotoxic effect of resveratrol, chrysin and morine on MCF-7 cells in MTT assay
In the present study, resveratrol showed inhibitory effect on MCF-7 cells proliferation, where the IC50 of resveratrol was foundto be 133.4 mM (81.45 to 218.5, CI 95%, R2 = 0.5949), (Figure 1). On the other hand, morin failed to show any cytotoxic effect on MCF-7 cells. However, a similar structured compound, chrysin showed significant cytotoxicity on MCF-7 cell lines. The IC50 was recorded and found to be 97.86 (66.23 to 144.6, CI 95%, R=0.9828), Figure 2).
Table 1: Cytotoxic effect of resveratrol, chrysin and morine on MCF-7 cells in MTT assay
| S. No. | Treatment groups | Concentration (mM) | Percentage cytotoxicity |
| Mean ± S.E.M. | |||
| 1 | Chrysin | 31.2 | 30.36 ± 0.10 |
| 62.5 | 52.09 ± 0.04 | ||
| 125 | 62.92 ± 0.12 | ||
| 250 | 58.06 ± 0.03 | ||
| 500 | 60.64 ± 0.07 | ||
| 2 | Morin | 31.2 | -23.87 ± 12.06 |
| 62.5 | -15.62 ± 10.44 | ||
| 125 | 5.09 ±10.48 | ||
| 250 | -27.83 ± 6.26 | ||
| 500 | -39.62 ± 5.28 | ||
| 3 | Resveratrol | 31.2 | -10.26 ± 0.76 |
| 62.5 | 35.51 ± 9.32 | ||
| 125 | 62.35 ± 1.58 | ||
| 250 | 75.47 ± 3.78 | ||
| 500 | 56.71 ± 9.92 |
These results suggest that, chrysin is superior to morin and resveratrol against MCF-7 cell lines in MTT assay. This could be linked with structural differences among the three, where the presence of 2,4-dihydroxyphenyl at 2ndposition of 3,5,7-trihydroxychromen-4-one resulted in loss of activity of morin as compared to chrysin. The chrysin molecule has aphenyl substitution at 2ndposition of the chomen-4-one ring is essential for the better cytotoxic activity.
Flavonoids are having multiple mechanism to prevent or cancer growth such as direct cytotoxic activity, inhibition of angiogenesis, anti-inflammatory and free radical scavenging activity. These flavonoids are well tolerated to human being as they are consumed as food. The combination of multiple mechanism could prevent the progression of cancer without producing toxicities. Hence, anticancer drugs can be developed using chrysin as lead molecule or flavonoid as pharmacophore.
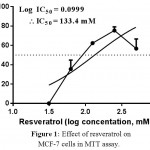 |
Figure 1: Effect of resveratrol on MCF-7 cells in MTT assay.
|
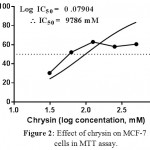 |
Figure 2: Effect of chrysin on MCF-7 cells in MTT assay.
|
Conclusion
The anticancer activity of flavonoid can be further studied taking chrysin as lead molecule. Chrysin is a good anticancer flavonoid compared to morin and resveratrol on MCF-7 cells by MTT assay.
Conflict of Interest
Authors declare that they do not have any conflict in publishing the data obtained.
Acknowledgement
Authors are thankful to the Manipal University, Manipal, Karantaka, India, for providing the facility and the resources to carry out the above research work.
Reference
- Harris TJ and Mccormick F, The molecular pathology of cancer, Nature reviews. Clinical oncology, 7, 2010, 251-65.
CrossRef - Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, and Fernandez-Salguero PM, The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle, Biochem Pharmacol, 64, 2002, 1375-86.
CrossRef - Karin M, Nuclear factor-kappaB in cancer development and progression, Nature, 441, 2006, 431-6.
CrossRef - Duthie GG, Duthie SJ, and Kyle JA, Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants, Nutrition research reviews, 13, 2000, 79-106.
CrossRef - Pandey KB and Rizvi SI, Plant polyphenols as dietary antioxidants in human health and disease, Oxidative medicine and cellular longevity, 2, 2009, 270-8.
CrossRef - Mohan A, Narayanan S, Sethuraman S, and Krishnan UM, Combinations of plant polyphenols & anti-cancer molecules: a novel treatment strategy for cancer chemotherapy, Anticancer Agents Med Chem, 13, 2013, 281-95.
CrossRef - Katsuya H and Tamura K, [Side effects of chemotherapy], Nihon rinsho. Japanese journal of clinical medicine, 73 Suppl 2, 2015, 39-44.
- Brechbuhl HM, Kachadourian R, Min E, Chan D, and Day BJ, Chrysin enhances doxorubicin-induced cytotoxicity in human lung epithelial cancer cell lines: the role of glutathione, Toxicol Appl Pharmacol, 258, 2012, 1-9.
CrossRef - Samarghandian S, Afshari JT, and Davoodi S, Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3, Clinics (Sao Paulo), 66, 2011, 1073-9.
CrossRef - Khoo BY, Chua SL, and Balaram P, Apoptotic effects of chrysin in human cancer cell lines, International journal of molecular sciences, 11, 2010, 2188-99.
CrossRef - Jin H, Lee WS, Eun SY, Jung JH, Park HS, Kim G, et al., Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB231 partly through suppression of the Akt pathway, Int J Oncol, 45, 2014, 1629-37.
CrossRef - Jayashree BS, Patel HH, Mathew NS, and Nayak Y, Synthesis of newer piperidinyl chalcones and their anticancer activity in human cancer cell lines, Research on Chemical Intermediates, 42, 2016, 3673-88.
CrossRef - Dinakaran VS, Jacob D, and Mathew JE, Synthesis and biological evaluation of novel pyrimidine-2(1H)-ones/thiones as potent anti-inflammatory and anticancer agents, Medicinal Chemistry Research, 21, 2012, 3598-606.
CrossRef - Paul P, Bansal P, Nayak PG, Pannakal ST, Priyadarsini KI, and Unnikrishnan MK, Polyphenolic fraction of Pilea microphylla (L.) protects Chinese hamster lung fibroblasts against gamma-radiation-induced cytotoxicity and genotoxicity, Environmental toxicology and pharmacology, 33, 2012, 107-19.
CrossRef - Mudgal J, Gowdra VS, Mathew G, Nayak PG, Reddy ND, Namdeo N, et al., Remedial effects of novel 2,3-disubstituted thiazolidin-4-ones in chemical mediated inflammation, Chem Biol Interact, 210, 2014, 34-42.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





