Manuscript accepted on : 04 September 2016
Published online on: --
Plagiarism Check: Yes
Bahram Fariborz Farsad1, Naser Hadavand1*and Sahar Masumi2
1Pharm.D, BCPS, Assistant Professor, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical sciences, Tehran, Iran.
2Pharmaceutical, Department of Clinical Pharmacy , Faculty of Pharmacy , Islamic Azad University , Pharmaceutical Sciences Branch , Tehran , Iran.
Corresponding Author E-mail: Hadavandn@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2290
ABSTRACT: Drug utilization studies may be one of the major design of optimizing the cost-effectiveness of treatment regimens and drugs that used to compare the costs and effects of alternative therapies. Human albumin (HA) is widely used for hypoalbuminemia and other diseases from deficiency of blood and liver factors. HA cost in the clinical setting is still controversial And Since use of albumin an established second line therapy process which is to be used in supportive drug for patients .on this study Trying to find first albumin protocol therapy cost was evaluated Then appropriate practice to be Alternate. A prospective, cross-sectional research was performed in “Shaheed Rajaei” Cardiovascular, Medical and Research Center during a 6-month continuous interval, in which, 300 patients have been evaluated .The study included patients who on a daily basis take albumin from the central pharmacy of Shaheed Rajaei hospital attaints time of receipt drug until discharge from hospital or termination of treatment were studied continuously. Demographic data including dose, intervals and durations have been registered, and then compared with international guidelines such as Data analyses have been done by SPSS 16 software. The results of this study indicated that among 300 in-patients who had prescribed albumin prescription assumed inappropriate for 281 patients (93.7%), The most common reason was incorrect indication of prescription without checking serum albumin level (56.39%) .base on this study the most reasons of albumin use were ascites and edema (57.9%),during the course of CABG(27.5%),hypoalbuminemia and hypoproteinemia(33.3%),acute nephritis(17.6%),hemodialysis(19.6%). also rate of infusion error was (16.5 %)and error related to dosages of order was ( 23.8 %).according to guidelines dosage of adminstration must be base on weight and serum albumin level that not be considred in intensive wards. According to data from this study suggests that You can see the protocol therapy based on the patient's needs Albumin consumption to reduce the cost of treatment and The lack of effectiveness of the drug, especially compared to other alternative medicines reduced.
KEYWORDS: Albumin Utilization; Efficacy; Cost
Download this article as:| Copy the following to cite this article: Farsad B. F, Hadavand N, Masumi S. Albumin Utilization Review to Evaluate the Efficacy and Cost, Perform as A Qualitative Study in Special Wards in Shaheed Rajaei Cardiovascular, Medical and Research Center. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Farsad B. F, Hadavand N, Masumi S. Albumin Utilization Review to Evaluate the Efficacy and Cost, Perform as A Qualitative Study in Special Wards in Shaheed Rajaei Cardiovascular, Medical and Research Center. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=16188 |
Introduction
Drug utilization studies may be one of the major design of optimizing the cost-effectiveness of treatment regimens and drugs that used to compare the costs and effects of alternative therapies . Serum therapy, patients in the ICU and CCU , one of the main challenges in the health system. Drug utilization evaluation studies Present valid reports, on the costs and effectiveness of drugs. Albumin is a regulator of circulating blood And it is create 80 and 70 percent of plasma oncotic pressure (1). The proper Indications of albumin involves the use of a variety of shocks (hemorrhagic and hemorrhagic bread), liver problems, burns, cerebral ischemia, cardiac surgery, neonatal hyperbilirubinemia, nephrotic syndrome, organ transplantation(2). But in compare with guidelines many cases the use of albumin in the hospital is not correct (3). Albumin as a major serum used in intensive care units in hospitals are not consumed properly, it is not a first-line treatment. The high volume consumer has always been associated with error and are prospective studies need to control and monitor the drug usage. Dur (Drug Utilization Review) is One way of assessing the quality and quantity of medication used(4). DUE programs or qualitative DUR studies during multilateral actions Information of rational drug use, collect, organize, analyze and report. albumin Due to its use in critically ill patients, high prices and ineffective in patients with indication for administration of albumin in them can not be seen, is a good choice for DUR studies. DUR studies in addition to controlling health care costs, is also the basis for drug development. However,in prospective studies that study was done in this way, You can quickly get to the complications and problems related to drug administration and immediately corrected (5,6). This study is designed to evaluate the pattern of albumin and its use in patients in intensive care in hospital .
Material and Method
This prospective study was performed at special wards of Shahid Rajaie Hospital, Tehran, Iran. adults older than 18 years in five different units including one ICU and four CCUs were included in this research.The program was conducted from March to August 2015.patiants with immediate emergency surgery,End-stage kidney disease (estimated GFR<20 mL/min – based on serum/ plasma creatinine), Medical history of MI within the past six months,Patients who have Cirrhosis and Signs or symptoms of acute MI on admission (Serum troponin level ≤0.1 u g/L) and Patients who have side effects of albumin were excluded from this study.we designed DUE forms that involved number of ordered and prescribed albumin vials, dosage and frequency of administration, indications based on guidelines and demographic information about the patients such as serum creatinine,smoking, history of underlying diseases such as heart or kidney or liver disease. some parameter such as ClCr ,total pro level,serum albumin and range of hepatic factors calculated. Appropriate or inappropriate albumin prescriptions were evaluated according to factors such as dosage or duration of prescribing albumin ,indication and total cost per patiant . then we analyses clinical data were by using SPSS statistical analysis software 16.
Result
Patients’ Demographics
During the study period, a total of 300 patients with a mean age of 56.35±14.7 years received albumin during a six months study . , 73.3% Of these patiants were male and 26.7% were female .The results of this study indicated that among 300 in-patients who had prescribed albumin prescription assumed inappropriate for 281 patients (93.7%), The most common reason was incorrect indication of prescription without checking serum albumin level (56.39%) .base on this study the most reasons of albumin use were ascites and edema (57.9%),during the course of CABG(27.5%),hypoalbuminemia and hypoproteinemia(33.3%),acute nephritis(17.6%),hemodialysis(19.6%). also rate of infusion error was (16.5 %) and error related to dosages of order was ( 23.8 %).according to guidelines dosage of adminstration must be base on weight and serum albumin level that not be considred in intensive wards.(table 1) show the proper indications of albumin adminstration.
Table 1 : The proper indications of albumin adminstration
| Indication | Description of use and dose of intravenous albumin | Rate of infusion | Choice of adminstration |
| Cardiopulmonary bypass surgery
(CABG) |
Solution 5% or 25% | Albumin 5%: Initial dose: 250 or 500 mL IV at a rate of 1 to 2 mL per minute in the absence of overt shock. The capacity of the administration set is the only limit in the exsanguinated patient. The rate of infusion and total volume administered are determined by the condition and response of the patient. The initial dose may be followed by additional albumin within 15 to 30 minutes if the response is inadequate. |
Postoperative
First Line: Crystalloids Second Line: nonprotein colloids Third Line: albumin |
| Hypovolemic shock and hemorrhagic shock | Initial dose : 250-500 ml ,12.5-25 gr solution 5% or 50-100 ml solution 25%
• Albumin 25% is contraindicated in hemorrhagic shock |
Reapeted dose every 15-30 minute
Dose adjustment should be based on the patient’s hemodynamic response |
First Line: Crystalloids
Second Line: nonprotein colloids Third Line: albumin |
| hypoproteinemia | Initial dose : 250-500 ml ,12.5-25 gr solution 5% or 50-100 ml solution 25%
Maintenance dose : 50-100gr/hr |
Albumin 5%: Initial dose: 250 or 500 mL IV at a rate of 1 to 2 mL per .The capacity of the administration set is the only limit in the exsanguinated patient. The rate of infusion and total volume administered are determined by the condition and response of the patient. The initial dose may be followed by additional albumin within 15 to 30 minutes if the response is inadequate. | · Albumin in patients with severe hypoalbuminemia to increase serum albumin concentrations not recommended based on current evidence.
· Instead, the cause of the underlying hypoalbuminemia should be identified and treated. |
| Hypoalbuminemia | Initial dose : 250-500 ml , ,12.5-25 gr solution 5%
Daily : 50-70 gr (200-300 ml ) solution 25% Max dose : 2 gr/kg/day |
5.7ml/h Infuse over 30-120 min; not to exceed 5-10 mL/min for 5% solution, 2 mL/min for 20% solution, and 2-3 mL/min for 25% solution | First Line: Calcitriol
FDA Approval: Adult, no; Pediatric, no Efficacy: Adult, Evidence favors efficacy Recommendation: Adult, Class IIb Second Line: Human albumin FDA Approval: Adult, yes; Pediatric, yes (12 to 16 years, Kedbumin(TM)) Efficacy: Adult, Evidence is |
The dose needed to obtain a serum albumin ≥ 2.5 g/dL is calculated using the following formula:
Dose (g) = [desired albumin concentration (2.5 g/dL) − actual albumin concentration (g/dL)] x plasma volume (0.8 x kg)
Demographic characterization of patients include general information , medical history ,duration of therapy and serum creatinine, has been presented in Table 2. The total number of used vials was 6950 and the patients received a mean number of 23.12 vials ranging from 1 to 68 vials. Our findings showed that albumin treatment course in our patients were 16.78 days.
Since the participating hospital was a tertiary center for cardiovascular diseases, the distribution of albumin prescriptions were mostly encountered in Intensive Care Unit (ICU) and Cardiovascular Care Unit (CCU). Our analysis showed that patients receiving albumin were admitted to the care units 61.2% in ICU and 38.8% in CCU.
Table 2: Patients’demographics and underlying diseases of the study participants
|
Variable (N=300)
|
Results* |
| Age, years
Weight
Sex
Mortality rate
|
56.39±14.07
69.83±8.62
26.7 % ; female 73.3 % : male
41.66 % : Discharge 58.33% : death
|
|
Length of stay(day)
|
16.78±14.25 |
| Admission ward | |
| Intensive care unit | 189 (61.2) |
| Cardiovascular care unit | 111 (39.8) |
| Underlying disease | |
| Admission during the course of CABG
Acute Nephritis
|
30 (27.5)
19.89(17.6)
|
| hypoalbuminemia and
hyponatremia
Hemodialysis
ascites and edema |
24.76(33.3)
28(19.6)
27(57.9) |
|
Number of used albumin vials
|
23.12±38.9 (1-68) |
|
Concurrent use of furosemide
|
69 (58.5) |
|
Lab values
|
|
| Serum albumin | 4.1±1.2 (2.2-4.5) |
| Total protein | 7.9±2.9 (3.7-8.3) |
| Serum creatinine | 1.8±0.7 (0.6-4.1) |
| Hematocrit | 42.5±6.1 (22.0-53.0) |
| AST | 89.8±157.2 (10-785) |
| ALT | 62.1±148.1 (7-791) |
| ALP | 299.6±173.6 (85-568) |
| CABG: Coronary Artery Bypass Graft;
ALT: Alanine transaminase AST: Aspartate transaminase ALP: Alkaline phosphatase *Mean ± SD (range) for continues variables; Frequency (%) for nominal variables. |
|
Table 1 also summarizes the mean plasma levels of AST (aspartate aminotransferase), ALT (alanine aminotransferase), alkaline phosphatase, albumin, total protein, hematocrit and creatinine of the study participants. Of these variables, although serum albumin is the most important index that may help in decision making for albumin use, only for 140 patients (46.66%) this value was measured. More interestingly, in our study, albumin levels showed a mean value of 4.1±1.2 g/dL (range 2.2- 4.5 g/dL) which is well above the accepted cutoff for any prescription (2.0 g/dL).
Table 3: Shows frequency of underlying disease in five special wards of shahid rajai hospital
| dieseases | Frequency % |
| Heart failure(n=87) | 29 |
| Diabetic(n=7) | 2.3 |
| Pulmunary edema(n=37) | 12.3 |
| Renal failure(n=38) | 12.7 |
| intestine Obstruction(n=8) | 2.7 |
| Pulmunary edema +TB[1](n=14) | 4.7 |
| Encephalopathy(n=9) | 3 |
| Endocarditis +TVR[2](n=8) | 2.7 |
| DVT[3](N=9) | 3 |
| DHF[4]+ Diabetic(n=9) | 3 |
| DVT+PE(n=4) | 1.3 |
| RF[5]+DHF+ Encephalopathy(n=8) | 2.7 |
| AV[6] modification(n=10) | 3.3 |
| MS[7](n=8) | 2.7 |
| DHF+HTN[8]+COPD[9](n=4) | 1.3 |
| PE+[10]RF(n=8) | 2.7 |
| Others (n=32) | 10.7 |
| total | 300 |
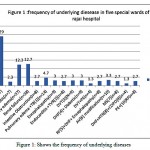 |
Figure 1: Shows the frequency of underlying diseases |
According to a study Albumin does not have any advantage over other alternative solutions
And it has more advers effect in patiants with underlying diseases and Albumin is more expensive than other alternative medicines patiants with immediate emergency surgery,End-stage kidney disease (estimated GFR<20 mL/min – based on serum/ plasma creatinine),Medical history of MI within the past six months,Patients who have Cirrhosis and Signs or symptoms of acute MI on admission (Serum troponin level ≤0.1 u g/L) and Patients who have side effects of albumin were excluded from this study because albumin can had more advers effect in these patiants.
Table 4: The rate of inappropriate albumin usage per patiants in the special wards of shahid rajai hospital
| DEPARTMENT | PERCENT OF PATIONT RECIVE ALBUMIN in last 6 mounths | PERSONAL AVERAGE USE OF ALBUMIN
In last 6 mounths |
| ICU A | 15.41 % | 22.69 |
| CCUC | 10 % | 15.46 |
| PCCUA | 5.12 % | 9.036 |
| CCUB | 8.45 % | 11.95 |
| CCUF | 9.02 % | 13.69 |
| Total rajaiee hospital | 10.54 % | 23.1 |
This table present the average number and percent of albumin used in Different ward ,based on this study the most incorrect use of albumin in ICU and the most cause of error in this part relaited to Non-performing lab tests properly and Lack of attention to the guidelines of appropriate indications of albumin adminstration.
Table 5: Cost Estimation by Improper use of the Albumin
| Albumin vials | February-march | April -march | May-April | June-May | July-June | July | Costs(dollar) | Costs(rial) |
| Post A | 146 | 194 | 91 | 43 | 78 | 80 | 237000 | 853,200,000 |
| ICU-A | 200 | 420 | 400 | 360 | 552 | 390 | 870750 | 3,134,700,000 |
| CCU-B | 240 | 135 | 198 | 220 | 178 | 148 | 419625 | 1,510,650,000 |
| CCU-C | 100 | 116 | 235 | 105 | 109 | 299 | 361500 | 1,301,400,000 |
| CCU-F | 255 | 370 | 200 | 380 | 430 | 251 | 707250 | 2,546,100,000 |
| total | 941 | 1235 | 1124 | 1108 | 1374 | 1168 | 2,596,125 | 9,346,050,000 |
Albumin is an expensive drug and Improper use of this drug Imposes large costs to patients and hospitals,according to this study 2,596,125 dollars estimated as extra total cost because of improper use of vials,also per patiant cost was 8653.75 dollars .
Discussion
Albumin as a major serum used in intensive care units in hospitals are not consumed properly, it is not a first-line treatment. If the non-protein crystalloid solutions and colloid can not be used or if the desired effect was seen after consumption albumin can be used . Incorrect administration of albumin are as noted belowHypoalbuminemia, using albumin as a source of nutrition, acute and chronic pancreatitis, support the patient’s blood pressure during dialysis , Ovarian hyperstimulation syndrome , Hepatorenal syndrome and increase the likelihood of drug efficacy (figure 2). These cases have been diagnosed inappropriate on the basis of the Consensus Exercise (7,8 ). The high volume consumer has always been associated with error and are prospective studies need to control and monitor the drug usage.
The role of albumin infusion in critically ill patients in the hospital during more than 20 years because of the high price of these products and their side effects and limited supply have been discussed. Many studies have evaluated the correct use of albumin in hospitals and academic centers that shows The use of albumin in many cases has not been properly, In some of these studies, after reforming consumption patterns and the use of appropriate guidelines The consumption of these products and costs are reduced. . In an observational study in 10 academic medical center in America proper use of albumin and used non-protein colloidal solutions were measured in 969 cases That only 24% good and 62% of the consumption of these products have been misplaced (9,10,11).
In two meta-analysis study that was conducted in 1998, it was shown that the consumption of non-protein albumin or colloids can lead to increased mortality in chronically ill patients . The first meta-analysis of 37 reviews about the RTC was that in 26 of these studies, colloids were compared with crystalloid. The results showed that fluid replacement in patients using colloidal solutions can increase 4%the absolute risk of mortality.
In the second meta-analysis of RCT 30 in the administration of albumin, or plasma protein derivatives with or without crystalloid solutions were compared, it was found that administration of albumin and 6% increased risk of death.
In a study conducted in Italy Martelli Antonietta and his colleagues, the impact of the Directions for Use of albumin in 2 hospital with third hospital without the use of albumin were taking appropriate guidelines, looked. The results of this study showed that during 2000 and 2001 at two hospitals that used the proper procedure, Albumin decreased, while consumption in the third hospital albumin increased over the years has shown. Finally, this study showed that the use of appropriate guidelines on the use of albumin can reduce the inappropriate use of these products and reduce costs (12,13,14).
In another study conducted in Belgium Somers A et al., Using albumin was studied in a University Hospital, Albumin in the hospital as an alternative to albumin (at very low levels of serum albumin), an increase of intravascular volume and maintain patient hemodynamically was used to improve tissue perfusion. In 1994, an agreement was made public in Belgium on the use of albumin and albumin Indications in which other alternatives were confirmed , After the preparation and communication of instructions and do DUE in the hospital for 4 months during the consumption of albumin in the hospital among The use of albumin in 90 patients (21%) of 115 cases were observed deviation from the instructions. Within 5 years of follow-up of this study showed that using the instructions on the amount of albumin consumption fell 50.1% from 1994 to 1999. . Finally came to the conclusion that the acceptance and use of guidelines on the use of albumin can reduce its consumption and costs.(15)
In a study of albumin DUE consumption in Brazil was evaluated at a hospital. In 99 patients and 33.1% of 1475 vials of used albumin was proper and albumin improper used was 61.8% and 4.6% of Disagreements and 0.4% were reported in unknown cause.(17,18)
An other study in Iran in 1999 and has been in the hospital Burn which shows The use of albumin in 97% of cases for improper and inconsistent with protocols ASHP (Directions for Use of albumin, non-protein crystalloid solutions and colloidal solutions, respectively).
The results of a study on the use of albumin DUE Acute care hospitals in New York found that 87%of 770 vials of albumin 25% of the 54 patients, had been prescribed inconveniently (19,20).
In a recent study it was done in Shariati Hospital In 1281 vials for 135 patients, only 411 bottles in 34prescription are suitable bases and is used on guidline based (21).
Our study also showed that In most patients , no Measurement of blood albumin levels prior to the administration of albumin are not done properly. The amount of 2.0 g / dL is a well accepted Criterion to define the cut off (22,23).
The results of this study indicated that among 300 in-patients who had prescribed albumin prescription assumed inappropriate for 281 patients (93.7%), The most common reason was incorrect indication of prescription without checking serum albumin level (56.39%) .base on this study the most reasons of albumin use were ascites and edema (57.9%),during the course of CABG(27.5%),hypoalbuminemia and hypoproteinemia(33.3%),acute nephritis(17.6%),hemodialysis(19.6%). also rate of infusion(16.5 %)and the dosages of order was incorrect( 23.8 %).according to guidelines dosage of adminstration must be base on weight and serum albumin level that not be considred in intensive wards.so all these errors Imposes a large cost to the hospital In the event that albumin has the same efficacy as other alternatives.
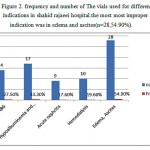 |
Figure 2: Frequency and number of The vials used for different indications in shahid rajaeei hospital.the most most improper indication was in edema and ascites(n=28,54.90%).
|
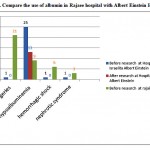 |
Figure 3: Compare the use of albumin in Rajaee hospital with Albert Einstein Hospital |
Conclusion
according to data from this study suggests that You can see the protocol therapy based on the patient’s needs Albumin consumption to reduce the cost of treatment and The lack of effectiveness of the drug, especially compared to other alternative medicines reduced. Drug Utilization Evaluation (DUE) is an effective Study Approach to identify inappropriate prescribing and to improve rational use of albumin and other expensive drugs.Reforming consumption patterns in most parts of the world is doing and Has helped to reduce the side effects and costs resulting from the indiscriminate use of medicines.For example in this below figure compare the use of albumin after with before doinng project.
References
- Howland WS, Schweizer O, Ragasa J, Jascott D.,et al. Colloid oncotic pressure and levels of albumin and total protein during major surgical procedures. Surg Gynecol Obstet. 1976 Oct;143(4):592-6.
- Heatherell M, Waggie Z, Purves L, et al. Correction of the anion gap for albumin in order to detect occult tissue anions in shock. Archives Dis Child. 2002;87:526–529.
CrossRef - Vermeulen Jr. LC, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med 1995; 155:373-9.
CrossRef - Jahangard-Rafsanjani Z, Javadi M, Torkamandi H, Alahyari S, Talasaz A, Gholami K. The Evaluation of Albumin Utilization in a Teaching University Hospital in Iran. IJPR 2011; 10(2):385-390.
- Debrix I, Combeau D, Stephan F, Benomar A, Becker A. Clinical practice guidelines for the use of albumin: results of a drug use evaluation in a Paris hospital. Pharm World Sci 1999; 21:11-6.
CrossRef - Boldt J, Brosch Ch, Röhm K, Lehmann A, Mengistu A, Suttner S. Is albumin administration in hypoalbuminemic elderly cardiac surgery patients of benefit with regard to inflammation, endothelial activation, and long-term kidney function? Anesth Analg 2008;107:1496-503.
CrossRef - Evans TW. Review article: albumin as a drug: biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther 2002; 16 (Suppl 5): 6-11
CrossRef - Mendez CM, McClain CJ, Marsano LS. Albumin therapy in clinical practice. Nutr Clin Pract 2005; 20.
CrossRef - Quinlan GJ, Martin GS, Evans TW. Albumin biochemical properties and therapeutic potential
Hepatology 2005: 41: 1211-9
CrossRef - Rinaldi S, Prinoth O. Proposta di linee guida per l’uso clinico delle immunoglobuline umane per somministrazione endovenosa (IVIG). Il Servizio Trasfusionale, 2002, 3: 12-9.
- Alejandra MM, Lansang MA, Dans LF, Mantaring JBV. Intravenous immunoglobulin for treating sepsis and septic shock. Cochrane Database Syst Rev 2002;
CrossRef - Antonietta Martelli, Paolo Strada, Ildefonso Cagliani, Giovanni Brambilla, Guidelines for the clinical use of albumin: comparison of use in two Italian hospitals and a third hospital without guidelines, Curr Ther Res Clin Exp. 2003 Nov; 64(9): 676–684
CrossRef - Vermeulen L.C., Jr., Ratko T.A., Erstad B.L. A paradigm for consensus: The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. 1995;155:373–379
CrossRef - Ragaller M.J., Theilen H., Koch T. Volume replacement in critically ill patients with acute renal failure. J Am Soc Nephrol. 2001;12(Suppl 17):S33–S39
- Somers A1, Bauters T, Robays H, Bogaert M, Colardyn F Evaluation of human albumin use in a university hospital in Belgium. Pharm World Sci. 2002 Jun;24(3):111-6
CrossRef - Mario tanzi, Melinda gardner, Michelle megellas, Steven lucio and Maryann resting.
Evaluation of the appropriate use of albumin in adult and pediatric patients Am J Health-Syst Pharm—Vol 60 Jul 1, 2003 - Debrix I, Combeau D, Stephan F et al. Clinical practice guidelines for the use of albumin: results of a drug use evaluation in a Paris hospital. Pharm World Sci. 1999; 21:11-6.
CrossRef - Vermeulen LC, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus: the University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions Ach Intern Med. 1995; 155: 373 – 379
- Offringa M. Excess mortality after human albumin administration in critically ill patients: clinical and pathophysiological evidence suggests albumin is harmful. BMJ. 1998; 317: 223 – 224.
CrossRef - Rubin H, Carlson S, DeMeo M, Ganger D, Craig RM. Randomized, double-bind study of intravenous human albumin in hypoalbuminemic patients receiving total parenteral nutrition. Crit Care Med. 1997; 25: 249 – 252
CrossRef - Adapted from the original published guidelines developed by the University Hospital Consortium and published in Archives of Internal Medicine GUIDELINES FOR USE OF ALBUMIN Revised – 2005 & 2010.
- Debrix I, Combeau D, Stephan F, Benomar A, Becker A. Clinical practice guidelines for the use of albumin: results of a drug use evaluation in a Paris hospital. Pharm World Sci 1999; 21(1):11-6.
CrossRef - Natsch S, vanLeeuwen SJ, de Jong R, Hekster YA. Use of albumin in intensive care unit patients–is continuous quality assessment necessary?. J Clin Pharm Ther 1998; 23(3):179-
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





