Manuscript accepted on : 19-Sep-2018
Published online on: 27-09-2018
Plagiarism Check: Yes
Girma Haile and Birhanu Babiye
Ethiopian Institute of Agricultural Research, National Agricultural Biotechnology Research Center, Microbial Biotechnology Program.
Corresponding Author E-mail: bbirhanu23@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2656
ABSTRACT: Enzymes are important in reducing both energy consumption and combating environmental pollution. Proteases are enzymes which catalyze the hydrolysis of protein molecules.Most of the tannery industries in Ethiopia use chemicals for dehairing that led great environmental and human health problem. The objectives of the present study were,to isolate potential protease producing bacteria from water sample collected from traditional leather processing waste water around Wukro maray;to extract the protease enzyme through SSF using cheap wheat bran, and evaluate the potential activity of the enzyme in leather dehairing. Water samples were serially diluted and 1ml of sample was spread on nutrient agar and kept at 370C for 24 hrs. Many colonies of bacteria were formed. The colony from C10-4 and G10-3 were taken by using inoculating loop for sub culturing to get pure colony. Then the pure cultured colony were inoculated into the 250 ml Erlenmeyer flasks containing substrate were fermented after 6 days incubation at 370C. The results of the unknown concentration of the crude protease enzyme showed successfully used as dehairing agent on hide. The results indicate that these bacteria isolate can be used as biotechnological tool for industrial purpose.
KEYWORDS: Bacteria; Leather Dehairing; Protease; Solid State Fermentation (SSF); Wastesq
Download this article as:| Copy the following to cite this article: Haile G, Babiye B. Extraction of Protease Under Solid State Fermentation Using Bacterial Isolates from Traditional Leather Processing Waste Water Found Around Wukro Maray. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Haile G, Babiye B. Extraction of Protease Under Solid State Fermentation Using Bacterial Isolates from Traditional Leather Processing Waste Water Found Around Wukro Maray. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31230 |
Introduction
Enzymes play crucial roles in different applications: in producing the food we eat, the clothes we wear, the drugs we need, the detergents we use, even in producing fuel for our automobiles, etc. Apart from use in various production processes towards greater efficiency, enzymes are also important in reducing both energy consumption and combating environmental pollution. Proteases are enzymes which catalyze the hydrolysis of protein molecules. Microbial proteases are among the most important, better studied enzyme groups since the development of enzymology. They are so far exploited as industrial catalysts in various industrial sectors, leather processing being the most interesting and potential area for large scale application (Abdullah, 2006).
Proteases are hydrolytic enzymes found in every organism to undertake important physiological functions. These include: cell division, regulating protein turnover, activation of zymogenic performance, blood clotting, lysis of blood clot, processing and transport of secretary proteins across membrane, nutrition, regulation of gene expression and virulence factors. Proteases differ in their specific activities, substrate specificities, pH and temperature optima and stability, active site, and catalytic mechanisms. All these features contributed in diversifying their classification and practical applications in industries involving protein hydrolysis.It also possess some characteristics of biotechnological interest due to which these have become the most important industrial enzymes (Barindraet al., 2006). Commercial proteases account for nearly 60% of the total industrial enzyme in the market (Udandi et al., 2009).
Neutralophilic and alkaliphilic microbial alkaline proteases possess a considerable industrial potential due to their biochemical diversity and stability at extreme pH environments, respectively (Moon et al., 1994). However, the demanding industrial conditions for technological applications and cost of protease production required continuous exercise for search of new microbial resources. Enzyme cost is also the most critical factor limiting wide use of protease for different applications. A large part of this cost is accounted for the production cost of the enzyme. Therefore, reduction in the production cost of enzymes could greatly reduce the cost ofthe enzyme. In submerged fermentation up to 40% of the total production cost of enzymes is dueto the cost of the growth substrate (Enshasy et al., 2008). In this regard, SSF which uses cheap agricultural residues have enormous potential in reducing enzyme production cost. However, studies on protease that are produced insolid state fermentation (SSF) by microorganisms are scarce in literature. As a result, it is of great importance to pursue such studies. This type of fermentation process also does not require highly caliber equipment and energy for agitation to provide oxygen.
Ethiopians are believed to have been practicing traditional leather processing since their ancient civilizations. This local knowledge has been transferred through generations and is being widely practiced these days to process leathers of cattle origin for making their shoes, clothes, beds, cushions and many other items primarily among the rural communities. They use small ponds usually on the sides of rivers and embed the leather for a period of time to remove hairs. However, very little has been done to promote this work through the application of biotechnology.
Most of the tannery industries in Ethiopia use chemicals for dehairing that led to great environmental and human health problem. The tannery pollutants are causing heavy damage to water resources, agriculture, fisheries, and so on. Since the shortage of income to import protease enzyme and lack of technology to produce this enzyme are the major problems that lead the industries to use chemicals like sodium sulfide. At present, there are few scientific reports available in Ethiopia on the potential microbial isolates that can be used in the process of dehairing. Therefore, there is a need to investigate the role and contribution of microorganisms during traditional leather processing.
Materialsand Methods
Time and Place of the Study
The study was conducted at Aksum, University College of Natural and Computational Science Department of biotechnology laboratory from March to June 2017 G.C.
Material and Chemical Required
Materials that were used to conduct the research were petri plate, conical flask, micropipette, inoculating loop, electronic balance, autoclave, test tube, hot plate, spatula,filter paper , fridge, measuring cylinder, beaker, laminar air flow, Bunsen burner, Ependroff tube, cotton, bottles, incubator, centrifuge, horizontal shaker, sample tube, ruler, scissor, and funnel.The chemicals used in the laboratory are like ethanol, MgSO4:7H2O, CaCl2, and distilled water.
Media Preparation
Nutrient agar was prepared from commercially available dehydrated base according to manufacture instruction. 22.4 gram of nutrient agar powder was weighted using a clean electronic balance then 800 ml of distilled water was added into bottle containing the measured of nutrient agar and shacked by hand to mix properly and covered with in a cotton, then the bottle sample was boiled on hot plate then placed in to autoclave for better sterilization for 15 min at 1210C.
Growth and Isolation of Bacteria
Samples were collected from traditional leather processing ponds/wastes (water from stagnate pond used for dehairing, from submerged goat and cow skin in stagnate pond) and was kept in sterile tubes in refrigerator, at 4°C until used. The sample collected from traditional leather processing pond was serially diluted (10-1 to 10 -5) andseeded on nutrient agar medium and after 24 hrs the bacteria was cultured and kept in refrigerator, until going to sub cultured. For sub culture goat sample (10-3) and cow sample (10-4) was selected and cultured by spread plate method on nutrient agar.
Preparation of Substrate
Wheat bran was collected from Dejen powder factory and it was sterilized by autoclaving at 121°C for 15 minutes.
Solid State Fermentation
Solid state fermentation medium containing (g/g): wheat bran, 10; MgSO4.7H2O, 0.02; CaCl2, 0.01; was prepared in a 250 ml Erlenmeyer flask, moistening agents was added in such a way to given final bran to moisture ratio of 1:1.5, thoroughly mixed, and autoclaved at 121ºC for 15 minutes. Then, the sub cultured (pure) bacteria was inoculated by using loop into each flask and, was incubated at 37°C for 5 days. From the fermented substrate, protease was harvested by soaking the fermented solid with ten volumes of distilled water per gram solid substrate (wheat bran), in shaking condition for 30 minutes at room temperature (Ikasari and Mitchel, 1996). At the end of the extraction, the suspension was hand squeezed through a double layered muslin cloth and the particulate materials clarified by centrifugation at 10,000 rpm for 5 minutes.
Analytical Methods
Test for Potential use in Enzymatic Dehairing
The sheep hides was washed and cut into 3×4 cm pieces. Two setswere placed into petri plate containing distilled water and tape water used as a control. The other set was placed into petri plate containing the enzyme solution by keeping liquid (ml) to gram hide proportion one to one (Najafi et al., 2005; Macedo et al., 2005; Malathi and Chakraborty, 1991) and it was incubated at 370C for 24 hrs. To assess dehairing extent, one petri plate at a time was taken for hair removal trial with fingers. The skin pieces after treatments was examined for dehairing time, dehairing extent.
Result and Discussion
Results
The study was conducted to extract industrial protease through solid state fermentation from traditional leather processing wastes, and it has the following results
Isolation of Bacteria
The bacteria were isolated from a sample that taken from traditional leather processing wastes through serial dilution and sub culture.
 |
Figure 1: Serial dilution and bacteria culture:
|
Serial dilution of from cow sample (a), serial dilution of from goat sample (b), serial culture of goat sample on nutrient agar (c), and serial dilution of cow sample on nutrient agar (d).
The sub culture were taken from 10-3 of Goat sample and 10-4 of Cow sample because those levels of serial dilution contains pure colony, and were spread on fresh nutrient agar by using spread plate methods.
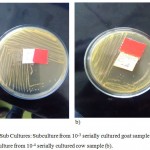 |
Figure 2: Sub Cultures: Subculture from 10-3 serially cultured goat sample (a), and sub culture from 10-4 serially cultured cow sample (b).
|
Solid State Fermentation
Pure cultured colony were inoculated into the 250ml Erlenmeyer flasks containing substrate were fermented after 6 days incubation at 370C, and then it was centrifuged.
 |
Figure 3: Fermentations: Fermentation by inoculating pure colony of goat sample (a), and fermentation by inoculating pure colony of cow sample (b).
|
Centrifugation
The fermented wheat bran with pure colony were centrifuged at 10000 rpm for 5 min. and it gives supernatant and two layer plate as shown below.
 |
Figure 4: Collected supernatant.
|
Enzymatic Activity on Sheep Hide Dehairing
The deharing results are shown in the table 1 and figure 5.
Table 1: Results of enzymatic activity on sheep hide.
| No | Sample | Temperature in 0C | Time in hrs | |||
| 6 | 12 | 20 | 24 | |||
| 1 | Cow crude sample | 37 | -ve | -ve | +ve | +ve |
| 2 | Distilled water | 37 | -ve | -ve | +ve | +ve |
| 3 | Goat crude sample | 37 | -ve | -ve | +ve | +ve |
| 4 | Tape water | 37 | -ve | -ve | -ve | -ve |
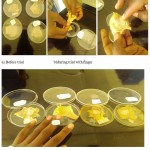 |
Figure 5: Enzymatic activities on sheep hide.
|
Discussions
In this study, microorganisms isolated from traditional leather processing water samples were screened for protease extraction. The microorganisms were protease positive. Enzyme production through SSF has enormous potential in reducing the cost of enzyme production. Since cheap wheat bran as a media substrate can be used, production cost could be minimized that indirectly minimize enzyme cost. Microbial growth medium for enzyme production at industrial scale takes about 30%-40% production cost (Enshay et al., 2008), by using wheat bran alone, appreciable amount of protease production can be achieved, implying presences of enough nutrients in wheat bran that supports the growth of the isolate and protease production. The selected isolate from isolate cow sample and goat sample grew well and produced protease under SSF. Thus, growth and extraction of appreciable amount of protease by isolate cow sample and goat sample under SSF offer better option for its large scale production. Many reports showed bacterial and fungus alkaline protease production lower at (≤ 250C) and moderate (300C-400C) temperatures, to mention are in Aspergillus and Bacillus strains preference of such temperature under SSF (Malthi and Chakraborty, 1991; Kumar and Takagi, 1999; Niadu and Davi, 2005; soarese et al., 2005).
Dehairing is also an important operation in tanneries conventionally practiced using lime and sodium sulphide (Thanikaivelan et al., 2004). In this process, the skin/hide is painted with sulphide which helps to reduce the disulphide bond that is responsible for attachment of hair keratin in epidermis. This brings about complete removal of hair, but the hair root remained within skin (Siva Subramanianet al., 2008). However, the use of alkaline protease has proven superior and efficient for selective removal of the non-collagen part of hide/skin (Kamini et al., 1999).
But in the present study was also give the false positive results on distilled water and the tape water was not de haired the hides. Results of enzymatic (crude protease enzyme) on sheep hide dehairing showed successful use of this enzyme as a dehairing agent. Complete dehairing of hide was achieved at 20 hr. Because of specificity to hydrolyse non-collagen protein part at hair roots in hide and the enzyme also was not purified, proteases are very important in shortening hide dehairing time and in production of high quality full gain leather having natural hair pores on the surface (Sivasubramanian et al., 2008). Cow/sheep hide is usually treated with dehairing chemicals in a drum for 24 hr (Thanikaivelan et al., 2004). Shortening of deharing time has been also reported, 20 hr for Aspergilus flavus protease by Malathi & Chakraborty (1991), and 9 hr for keratinases of Bacillus subtilis S14 by Macedo et al., (2005). Thus, the centrifuged cow and goat fermented sample produced crude protease has a potential to substitute environmentally objectionable dehairing chemicals for hide/skin dehairing in leather industries and for production of quality leather products.
Conclusions
Proteases are one of the most important groups of industrial enzymes with considerable application in the animal feed processing, leather industry, medical activity, detergent additive, protein hydrolysis, silver recovery, management of wastes and other sectors. In this study, protease was successfully produced by bacteria isolated from traditional leather processing ponds for the purpose of dehairing.
Generally the crude protease separated by centrifuge and its enzymatic activity on sheep hide was successfully used as deharing agents.
References
- Abdullah F. M. The production of extracellular protease using Bacillus subtilis effect of temperature and agitation speed. MSc Thesis. University Collegeof Engineering and Technology. Malaysia. 2006;46.
- Agrawal D., Patidar P., Banerjee T and Patil S. Alkaline protease production by the soil isolate of Beauveri felin under solid state fermentation condition: parameter optimization and application to soya protein hydrolysis. Biochem. 2004;40:1131-1136.
- Aguilar C. N., Gutierrez-Sancez G., Rado-Barragan P. A., Rodriguez-Herrera R., Martinez-Hernandez J. L and Contreras-Esquivel J. C. Perspectives of solid state fermentation for production of food enzymes. J.Biochem. & Biotech. 2008;4:354-366.
CrossRef - Gessesse A., Hatti-kaul R., Berhanu A. G and Mattiasson B. Novel Alkaline protease from alkaliphilic bacteria grown on chicken feather. E Microb. Technol. 2003;32:519-527.
- Barindra S., Debashish G., Malay S and Joydeep M. Purification and characterization of a salt solvent detergent and bleach tolerant protease from a new gamma Proteo bacterium isolated from the marine environment of the Sundarbans. Process Biochem. 2006;41(1):208-215.
CrossRef - Barrios-Gonzalez J.,Fernandez F. J., Tomasini A and Mejia A. Secondary metabolites production by solid state fermentation. J. microbial. 2005;1:1-6.
- Chiplonkar J. M., Gangodkar S. V., Wagh U. V., Ghadge G. D., Rele M. V and Srinivasan M. C. Applications of alkaline protease from Conidiobolus in animal cell culture. lett. 1985;7:665-668.
- Crout D. H., Macmanus D. A., Rica J. M., Singh S., Critchley P and Gibson W. T. Biotrans formation in the peptide and carbohydrate fields. Pure & Appl.chem. 1992;64:1079-1084.
CrossRef - Silva D. M. Enzymatic Treatment of Wool with Modified Proteases. D Thesis. University of Minho. 2005.
- Enshasy E. H., Abuol-Enein A., Helmy S and Azaly E. Optimization of the industrial. 2008.
- Freddi G., Mossotti R and Innocenti R. Degumming of silk fabric with several proteases. J Biotechnol. 2003;106:101-112.
CrossRef - Fujiwara N., Yamamato K and Masui A. Utilization of thermostable alkaline protease from an alkaliphilic thermophile for the recovery of silver from used x-ray film. Fermt.Bioeng. 1991;72:306-308.
CrossRef - Gençkal H. Studies on Alkaline Protease Production from Bacillus MSc Thesis. İzmir Institute of Technology, İzmir, Turkey. 2004;98.
- Gupta R., Beg Q. K and Lorenz P. Bacterial alkaline proteases molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 59:15–32.
- Gupta R., Beg Q. K and Lorenz P. Bacterial alkaline proteases molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 59:15-32.
- Gupta R., Beg Q. K.,Khan S and Chauhan B. Anoverview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol. 60(4):381-95.
- Guzman F., Brberis S and Illanes A. Peptide synthesis. Chemical or enzymatic. 2007;10:179-314.
- HŐlker U and Lenz J. Solid-state fermentation-are there any biotechnological advantages? Opin. Microbiol. 2005;8:301–306.
CrossRef - Horikoshi K. Alkaliphiles some applications of their products for biotechnology. Mol. Biol. Rev. 1999;63:735–750.
- Ikasari L and Mitchell D. A. Leaching and characterization of Rhizopus oligosporus acid protease from solid-state fermentation. Microb. Technol. 1996;19:171-175.
CrossRef - N. Enhanced production of thermo stable bacterial proteases and their applications. PhD. Disertation. University of Punjab. Pakistan. 2008;158.
- Kamini N. R., Hemachander C., Mala J. G. S and Puvanakrishnan R. Microbial enzyme technology as an alternative to conventional chemicals in leather industry. Sci. 1999;77:80-86.
- Kumar C. G and Takagi H. Microbial alkaline proteases from bio-industrial viewpoint. Biotechnol. Adv. 1999;17: 561–594.
CrossRef - Kumara M. S. Kashyap N. S., Vijay R., Rahul T., Anuradha M. Production and optimization of extra cellular Protease from bacillus sp. Isolated from soil. International Journal of Advanced Biotechnology and Research. 3:564-569.
- Kwon Y. T., Kim J. O., Moon S. Y., Lee H. H and Rho H. M. Extracellular alkaline protease from alkaliphilic Vibrio metschnikovii strain RH 530. Biotechnol Lett. 1994;16:413–418.
CrossRef - Macedo A. J., Silva D. W. O., Gava R., Driemeier D., Henriques J. A. P and Termignoni C. Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl. Env. Microbiol. 71:594-596.
CrossRef - Malathi S and Chakraborty R. Production of alkaline proteases by a new Aspergillus flavus isolate under solid state fermentation conditions for use as a depilation agent. Environ. Microbiol. 1991;57:712-716.
- Maurer K. Détergent protéases. Opin. Biotechnol. 2004;15:330–334.
CrossRef - Moon S. Y., Oh T. K and Rho H. M. Purification and characterization of an extra cellular alkaline protease from Bacillus subtilis RM Korean Biochem.J. 1994;27:323-329.
- Mukhtar M and Ul-Haq I. Production of alkaline protease by Bacillus subtilis and its application as a depilating agent in leather processing. J.Bot. 2008;40:1673-1679.
- Nadeem M. Biotechnological production of alkaline protease for industrial use. PhD Thesis. University of Punjab, Lahore, Pakistan. 2009;208.
- Najafi M. F., Deobagkar D and Deobagkar D. Potential applications of protease isolated From Pseudomonas. aeruginosa E J .Biotechnol. 2005;8:197-203.
- Nakiboglu N., Toscali D and Yasa I. Silver recovery from waste photographic films by an enzymatic method. TurkJ Chem. 2001;25:349-353.
- Niadu K. S. B and Devi K. L. Optimization thermostable alkaline protease production from species Bacillus using rice bran. Afr. J.Biotechnol. 2005;4:724-726.
CrossRef - Nirmal N. P., Shankar S and Laxman R. S. Fungal Proteases: An Overview. J. Biotech and Biosci. 1(1):1-40.
- Pandey A., Selvakumar P., Soccol C. R and Nigam P. Solid state fermentation for the production of industrial enzymes. sci. 1999;77:149-162.
- Pérez-Guerra N., Torrado-Agrasar A., López-Macias C and Pastrana I. Main characterstics and applications of solid substrate fermentation. J.Envir. Agric. Food chem. 2003;2:343-350.
- Production of alkaline protease by Bacillus licheniformis in different production. Aust. J.Bas. Appl.Sci. 2:583-593.
- Rao M. B., Tanksale A. M., Ghatge M. S and Deshpande V. V. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 1998;62:597-635.
- Singhal P., Nigam V. K and Vidyarthi A. S. Studies on production characterization and applications of microbial alkaline proteases. International Journal of Advanced Biotechnology and Research. 3(3):653-669.
- Sivasubramanian S., Manohar M and Puvanakrishnan R. Mechanism of enzymatic dehairing of skins using a bacterial alkaline protease. Chemosphere 70:1025-1034.
CrossRef - Soarese V. F., Castilho L. R., Bon E. P. S and Freire D. M. G. High yield Baccilus subtilis for protease production by solid state fermentation. Human press. 2005;311-319.
- Sumantha A., Larroche C and Pandey A. Microbiology and Industrial Biotechnology of Food-Grade Proteases: A Perspective. Food Technol. Biotechnol. 2006;44(2):211–220.
- Thanikaivelan P., Rao J. R., Nair B. U and Ramasami T. Progress and recent trends in biotechnological methods for leather processing. Biotechnol. 2004;22:181-188.
- Thanikaivelan P., Rao J. R., Nair B. U and Ramasami T. Recent trends in leather making: processes problems and pathways. Rev. Env. Sci.Technol. 2005;35:37-79.
CrossRef - Udandi B., Rajendran R., Palanivel K. V. S and Manoharan M. J. Optimization of Protease Enzyme Production Using Bacillus Isolated from Different Wastes.Bot. Res. Intl. 2009;2(2):83-87.
- Underkofler L. A., Barton R. R and Rennert S. S. Production of microbial enzymes and their applications. Microbiological Process Report. 1958;6:212-221.
- Vishwanatha K. S. Acid protease from Aspergillus oryzae Structure stability and enhancement of the activity by physical chemical and molecular biological approaches. PhD. Thesis. Central Food Technological Research Institute. Karnataka, India. 2009;266.
- Yust M., Pedroche M., Megías J. C., Girón-Calle J., Alaiz M., Millán F and Vioque J. Rapeseed protein hydrolysates a source of HIV protease peptide inhibitors. Food chem. 2004;87:387-392.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





