Manuscript accepted on : 09 March 2018
Published online on: --
Use of Different Agroindustrial Waste and Produced Water for Biosurfactant Production
Emilianny Rafaely Batista Magalhães1, Francinaldo Leite Silva1,2, Magna Angélica dos Santos Bezerra Sousa1 and Everaldo Silvino Dos Santos1
1Laboratory of Biochemical Engineering, Chemical Engineering Department at Federal University of Rio Grande do Norte (UFRN), Natal/RN, Brazil. 2Instituto Federal de Educação Ciência e Tecnologia da Paraíba (IFPB) - Campus Picuí, Picuí/PB, Brazil.
Corresponding Author E-mail: everaldo@eq.ufrn.br
DOI : http://dx.doi.org/10.13005/bbra/2604
ABSTRACT: The high and increasing environmental concern about chemical surfactants triggers attention to more eco-friendly compounds, which are capable of presenting low toxicity and biodegradable nature. This study has evaluated the emulsifying potential of broths obtained from production of rhamnolipids by Pseudomonas aeruginosa AP 029/GLVIIA using different agro-industrial wastes as carbon source, and the influence of using produced water in the culture medium then acting as an inducer. There were used residues of coconut, cashew, sugar cane, carnauba, and moringa seeds. These materials were chemically characterized and used in the production of biosurfactant broths. The emulsifying activity and the surface tension were used as indirect analysis for determining the production of rhamnolipids on biosurfactant broth. For coconut and cashew residues the pH emulsion was observed along time at different temperatures such as 4ºC, 25ºC and 40ºC The emulsification index of all broths were assessed with and without presence of produced water in the culture medium and has indicated the stability of the emulsion along time. Coconut and cashew residues showed a better stability of oil/water emulsion than the other ones, evidencing their potential surfactants. The lowest surface tension about 40 N/m and the highest contact angle (approximately 69 o) were observed for the coconut residue.
KEYWORDS: Agro-Industrial Waste Biosurfactant; Emulsion; Pseudomonas Aeruginosa; Rhamnolipids;
Download this article as:| Copy the following to cite this article: Magalhães E. R. B, Silva F. L, Sousa M. A. D. S. B, Santos E. S. D. Use of Different Agroindustrial Waste and Produced Water for Biosurfactant Production. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Magalhães E. R. B, Silva F. L, Sousa M. A. D. S. B, Santos E. S. D. Use of Different Agroindustrial Waste and Produced Water for Biosurfactant Production. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29296 |
Introduction
The emulsion is a composed system of two immiscible fluids, which dispersed phase is finely divided and distributed in continuous phase.1 The agents responsible for maintaining the stability of emulsion are called emulsifiers or surfactants whose amphilic nature is capable of reducing surface tension between two immiscible phases and consequently of decreasing emulsion instability.2,3 These compounds, which represent a major market production of over 15 million tons per year worldwide, correspond to one of most versatile groups of chemicals used industrially, with applications in formulation of cleaning products, detergents and cosmetics, in food and cosmetics industries and environmental remediation.4,5
Initially, surfactants were produced exclusively from renewable resources such as vegetable oils or animal fats. That is why most of what is available currently derived from petrochemical sources.1 Research involving use, production and purification of biosurfactants has intensified due to economic competitiveness and increasing demands for environmental correct and eco-friendly products. Especially for microbial surfactants, they present many environmental advantages, such as biodegradability, low toxicity, produced from renewable sources, and similar or superior performance when compared to chemical surfactants.4,6–9
Bacteria of genus Pseudomonas are well studied due to their biosurfactant synthesis power. The biosurfactants frequently produced by Pseudomonas aeruginosa are mono-rhamnolipids and di-ramololipids.9 These biosurfactants are derived from rhamnose, which is one of lipopolysaccharide (LPS) components present in cell wall of P. aeruginosa. Biosynthesis and ratio of rhamnolipid types are affected by the environmental and nutritional conditions of microbial growth medium.10
In study developed by Chin-chi Lai et. al,11 rhamnolipids produced from Pseudomonas aeruginosa were used as surfactants in remediation of highly contaminated soils with total petroleum hydrocarbons (TPH). They have showed better efficacy of TPH removal around 63% when compared to synthetic ones, such as Triton X-100 and Tween 80, with efficiency of 40% and 35%, respectively.
In rhamnolipid production, besides their costs for downstream processing and low productivities, relatively high prices of raw materials used as carbon sources, which accounts for around 50% of total process cost, turn more difficult economically rhamnolipid production on industrial scale. Thus, use of low cost and renewable carbon source may represent an important saving in process of biosurfactants production.2,12,13 Several studies have already been carried out to evaluate effect of different carbon source on rhamnolipid production, such as soybean oil, sunflower oil and agricultural refinery waste of palm oil.14,15 On the other hand, use of agro-industrial residues in rhamnolipids production has still been few explored even though they show expressive availability and low cost. In this context, this work aims to evaluate the emulsifying potential in an oil/water emulsion (O/W) of broths obtained in process of rhamnolipid production by Pseudomonas aeruginosa AP 029/GLVIIA from different agro-industrial wastes used as carbon source as well as the influence of produced water from petroleum industry in culture medium.
Material and methods
Agro-industrial wastes
The carnauba straw (Copernicia prunifera) was donated by Non-Governmental Organization (NGO) “Carnaúba Viva” located in Assu-RN, Brazil. Usina Estivas located in Arês (RN), Brazil donated the sugarcane (Saccharum officinarum) residue. The green coconut residue (Cocos nucifera) was purchased in Ponta Negra-Natal (RN), Brazil. The cashew peduncle residue (Anacardium occidentale) was obtained by CIONE chestnut company located in Fortaleza (CE), Brazil. The Moringa oleifera seeds were collected in Natal (RN), Brazil, whose tree is in the geographical coordinates 5°48’38.5″ South latitude and 35°12’27.6″ West longitude. Such wastes were washed with water with the purpose of removing particulates from all materials, they were dried at 70°C on drying oven for 48 hours. Then they were ground in a knife mill (Willye type mill, TE-680, Tecnal, Brazil), sieved at 20 mesh strainer and stored at room temperature (25°C).
Chemical-physical characterization
The cellulose, hemicellulose, lignin, ash, moisture contents, and extractives were evaluated from both the untreated and pretreated biomass according to NREL (National Renewable Energy Laboratory – EUA).16,17
Microorganism and Biosurfactant Broths
The P.aeruginosa strain AP 029/GLVIIA was isolated from petroleum field and maintained at Biochemical Engineering Laboratory of Federal University of Rio Grande do Norte (Natal-Brazil). Prior to use, cells were maintained in PCA medium at 5°C.18 The inoculum was obtained with 100 mL of medium (5.0 g/L peptone, 3.0 g/L yeast extract and 5.0 g/L NaCl) prepared in 250 mL Erlenmeyer flasks. The pH solution was adjusted to 7.0 using NaOH.19 Then, the flasks were sterilized. Subsequently, the P. auruginosa cells were incubated on shaker (TE-421, Tecnal, Brazil) at 200 rpm (38°C) for 24 hours. For the production of biosurfactant broth, 3 mL of inoculum was added to 97.0 mL of culture medium (18.0 g/L glucose, 1.0 g/L MgSO4 .7H2O, 1.1 g/L Na2 HPO4 .7H2O , 1.5 g/L K2HPO4, 2.0 g/L NaNO3, and 0.1 g/L FeSO4 .7H2O, the pH was adjusted to 6.5 using NaOH). Nex the flasks were held at 200 rpm (38°C) for 72 hours.20 For cultivation trial where different biomass was used, 18.0 g/L of glucose was substituted on culture medium by 2% (w/v) of one of biomass in each culture. In order to evaluate the effect of produced water, distilled water was replaced by produced water from oil and grease content (OGC) of 150 ppm on preparation of culture medium. After culture time, cells were centrifuged at 3,500 rpm for 15 minutes.
Broths used in this study were produced using differents agro-industrial wastes to induce rhamnolipid production with and without presence of produced water in culture medium as presented in Table 1.
Table 1: Agro-industrial wastes used as carbon source to induce rhamnolipid production with and without presence of produced water in culture medium.
| Abbreviature of broths | Definition |
| CA | Sugarcane |
| CAAP | Sugarcane with produced water |
| CAJ | Cashew |
| CAJAP | Cashew with produced water |
| CAR | Copernicia prunifera |
| CARAP | Carnauba with produced water |
| CO | Coconut |
| COAP | Coconut with produced water |
| GLI | Glucose |
| GLIAP | Glucose with produced water |
| MO | Moringa oleifera |
| MOAP | Moringa oleifera with produced water |
Emulsifying Activity (EA) and Emulsification Index (EI)
The production of biosurfactants was assessed by oil in water emulsification (O/W) activity. Therefore, 3.5 mL of cell free culture broth were mixed with 2 mL of toluene into a tube. Initially, the optical density was measured at 610 nm using a spectrophotometer in order to obtain the blank value. Thereafter, each tube was shaken vigorously on vortex shaker for 2 minutes. After 1 h, optical density was again measured at 610 nm.21 Then, emulsification activity was determined by Equation 1
EA = (Abst1-Abst0) Χ D Eq.1
where:
EA = Emulsifying Activity;
Abst1= Absorbance 1 hour after shaking;
Abst0 = Absorbance 0 time;
D = Dilution of sample.
Emulsification index was determined by adding toluene to cell-free culture broth (2 mL each) in test tubes followed by high speed vortexing for 2 minutes. The tubes were allowed to stand for 24 hours and emulsification index was calculated by Equation
EI = (EA – AT) Χ 100 Eq.2
where,
EI= Emulsification Index
EL= (Emulsification layer height)
AT= Total mixing height
In order to monitor the stability of emulsion, emulsification index was measured at every 24 hours for 15 days after test.
Surface Tension and Contact Angle
Surface tension of emulsions was determined by dropping method using a tensiometer (Phoenix 150, Surface Electro Optica, Canada) and Surfaceware8 software (version 10.11). Contact angle was measured on a rectangle glass plate using same equipment.
Microscopic Analysis of Emulsions
Stability of emulsions was also evaluated from bubbles size. It was counted the bubbles and has measured their diameters with an Olympus BX51 microscope and analySIS software (Olympus-Tokyo, Japan). Bubble size distribution was obtained using the software Statistica 7.0.22
Influence of Temperature on the pH of raw Broths
Oil/water emulsions were submitted to three different temperatures: refrigerator (4°C), ambient temperature (25°C) and biological incubator (40°C). The pH was measured shortly after preparation of emulsions, after 3 days and 7 days.
Statistical Analysis
Statistical analysis were performed by using Statistica 7.0 software 22 using the Tukey test for three independent samples at 5% level of significance (p < 0.05).
Results and Discussion
Chemical-Physical Characterization
A variety of wastes can be used for the production of biosurfactant, especially rhamnolipids, such as vegetable oils, sugars and glycerol.2 In this study, according to characterization of residues presented in Table 2, it was observed that agro-industrial wastes used are mostly residues of lignocellulosic characteristic, that means, they have in their chemical constitution a predominance of cellulose, hemicellulose and lignin, but M. oleifera seeds. The latter was characterized as an oleaginous material, with rich composition in extractives and poor in lignin and cellulose.
Table 2: Chemical characterization of the agro-industrial wates (dry basis) used as carbon source for the production of crude broth containing biosurfactant.
| Carbon source | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Extractives (%) | Ashes (%) |
| Carnauba (CAR) | 23.96 ± 0.75 | 11.84 ± 0.82 | 32.79 ± 0.91 | 11.62 ± 0.45 | 7.69 ± 0.05 |
| Cashew (CAJ) | 21.02 ± 0.31 | 11.50 ± 1.13 | 45.84 ± 1.28 | 10.75 ± 0.86 | 1.04 ± 0.02 |
| Coconut (CO) | 36.23 ± 0.09 | 23.79 ± 0.31 | 30.23 ± 0.12 | 8.50 ± 0.04 | 0.86 ± 0.04 |
| Moringa (MO) | 12.02 ± 0.81 | 14.61 ±0.43 | 5.37 ± 0.98 | 69.94 ± 1.40 | 3.60 ± 0.02 |
| Sugarcane (CA) | 39.23 ± 5.49 | 25.29 ± 1.13 | 18.82 ± 0.01 | 9.74 ± 0.21 | 3.52 ± 0.21 |
Emulsifying Capacity of Produced Raw Broth
According to results presented in Figures 1 and 2, it is possible to state that, among in natura carbon sources, CO, COAP, CAJ and CAJAP samples were able to produce broths containing biosurfactant with emulsifying power and capacity of stabilizing the emulsion. In all these broths, they have showed better characteristics than systems with glucose, that is commonly used for rhamnolipids production by P. aeruginosa.23,24 These promising performances of CO, COAP, CAJ and CAJAP samples may be directly related to relevant amounts of cellulose, hemicellulose and lignin of coconut and cashew residues (Table 2). As shown in previous studies, bacteria of genus Pseudomonas demonstrate the ability to synthesize lignocellulolytic enzymes.25–27 Thus, it is feasible that P. aeuroginosa uses these lignocellulosic residues to consume assimilable sugars as glucose. Lignocellulosic residues require enzymes cellulases, hemicellulases and xylanases with purpose of hydrolyzing them into glucose. It is expected that P. aeruginosa uses this mechanism to obtain greater emulsifying power in CO, COAP, CAJ and CAJAP. However, further investigations should be carried out for the strain used in this study to elucidate the mechanism. Another factor to be considered is the possibility of free glucose existence in these broths, which may favor the bacterial growth.2
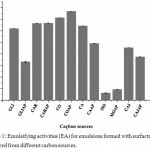 |
Figure 1: Emulsifying activities (EA) for emulsions formed with surfactant broths produced from different carbon sources.
|
Carbon sources: GLI, GLIAP, CAR, CARAP, CO, COAP, CA, CAAP, CAJ, CAJAP, MO, MOAP. All analyzes were performed in triplicate.
 |
Figure 2: Emulsification indices (EI) for emulsions formed by toluene-water-crude broth for each source of carbon used, varying with time (t).
|
Carbon sources: : GLI, GLIAP, CAR, CARAP, CO, COAP, CA, CAAP, CAJ, CAJAP, MO, MOAP. All analyzes were performed in triplicate.
It is important to notice that M. oleifera seeds, with and without presence of produced water in culture medium (MO and MOAP), presented lower emulsifying activity when compared to others. The possible reason may be associated to low cellulose content into this material and, consequently, to low production of biosurfactant.
Surface Tension and Contact Angle
Based on former results, GLI, GLIAP, CO, COAP, CAJ and CAJAP samples were selected for stress and contact angle analysis. It is known that surfactant effectiveness is determined by its ability to reduce surface tension of emulsion, defined as free surface energy per area unit required to bring a molecule from interior bulk to surface [28]. The rhamnolipids reduce surface tension of water around to 31 mN/m.24 In this study, all samples that have presented high emulsification index had a lower surface tension value than water (< 72 mN / m) (Table 3). That indicates the ability of these surfactant broths to decrease interface strength of mixture between two immiscible components, allowing its emulsion. Similar results were obtained by Gudiña et al. (2015) and Radzuan et al. (2017).4,15 The CO sample, which uses coconut as an inducing substrate for rhamnolipid production without presence of produced water, was the one that stood out the most in emulsification index. Moreover it also has presented contact angle higher than other samples, which means a lower wettability and, thus, a lower adhesion to measured surface.
Table 3: Properties of emulsions formed using broths obtained from the different carbon sources
| Parameter | GLI | GLIAP | CO | COAP | CAJ | CAJAP |
| Superficial tension (mN/m) | 45.10± 1.05 | 42.61±0.77 | 39.82±0.67 | 53.18±1.76 | 49.72±1.15 | 55.50±0.00 |
| Contact Angle (°) | 49.50±1.77 | 34.55±0.85 | 68.53±1.77 | 57.15±0.50 | 50.85±1.13 | 42.82±2.19 |
Formation and Stability of Emulsions
The stability of formed emulsions from crude broths produced by P. aeruginosa AP 029/GLVIIA using nutritious media containing CO, COAP, CAJ and CAJAP as carbon sources were studied due to their better performance, even when compared with glucose as carbon source. Results of emulsion stability are showed in Figures 3 and 4. It was observed when using CO broth to produce emulsion utilizing toluene as dispersing phase that, after 24 hours of preparing emulsions, bubbles had an average diameter of 12.63 μm with majority diameter of bubbles being 8 μm (Figure 3A). After 15 days of formation of emulsion, the mean diameter of bubbles was 35.02 μm and most bubbles had a diameter among 25 and 35 μm (Figure 3B).
It was noted that the mean diameter of bubbles was 20.13 μm in 24 hours and most diameter of bubbles had 7 μm (Figure 3C) concerning stability of formed emulsions with crude COAP-produced broth. After 15 days of experiment, the average diameter of bubbles became 28,14 μm, being the most diameter of bubbles of 17, 27 and 45 μm (Figure 3D). It was verified that COAP emulsions are less stable than CO because their variation of bubble diameter is greater.
 |
Figure 3: Microscopic visualization of the emulsion using A) coconut shell(CO) bagasse as carbon source for the production of biosurfactant through
|
P. aeruginosa after 24h and bubble diameter; B) CO after 15 days and distributions of bubble sizes; C) emulsion using coconut husk bag and produced water (COAP) as carbon source after 24h and bubble size distribution; D) emulsions produced with COAP after 15 days and distributions of bubble sizes.
The study of emulsions from crude broths produced by CAJ and CAJAP as carbon sources, in general, has presented larger diameter bubbles than emulsions using CO and COAP. Emulsions using CAJ had an average diameter of 27.68 μm, most diameter of which are 25 μm after 24 hours (Figure 4A). At the end of 15 days of produced emulsion with CAJ, the mean diameter of bubbles was of 33.11 μm (Figure 4B). On the other hand, CAJAP presented a majority diameter of bubbles of 47 μm after 15 days. (Figure 4D).
 |
Figure 4: Microscopic visualization of the emulsions. A) cashew peduncle (CAJ) as carbon source for the production of biosurfactant through
|
P. aeruginosa after 24h and bubble diameter; B) CAJ after 15 days and distributions of bubble sizes; C) emulsion using cashew peduncle and produced water (CAJAP) as a source of carbon after 24h and distribution of bubble size; D) emulsions produced with CAJAP after 15 days and distributions of bubble sizes.
Analyzing those data, it could figure out that the produced water had a positive influence in the biosurfactant production by coconut as carbon source, because there was a lower variation in their bubbles mean sizes. Unlike behavior was observed when using cashew, where the use of produced water increased this variation. In all this cases, bubbles mean sizes when using coconut were lower than cashew.
Influence of Temperature on pH of Emulsions
According to Lovaglio et. al,29the rhamnolipids have demonstrated an excellent emulsifying activity at pH values between 5 and 9. In these circumstances, this study has evaluated pH variation of emulsions containing biosurfactant at three different temperatures (4°C, 25°C and 40°C) during 7 days (Figure 5). There were used the emulsions produced by CO, COAP, CAJ and CAJAP, which have presented better performance in emulsification index in the course of 15 days (Figure 2). GLI and GLIAP were also used as control once that glucose is usually utilized as carbon source in culture media for rhamnolipid production by P. aeruginosa AP 029/GLVIIA.18,20
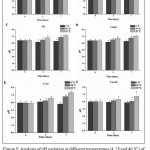 |
Figure 5: Analysis of pH variation at different temperatures (4, 25 and 40°C) of emulsions. A) CO (coconut) and B) COAP (coconut and produced water), C) CAJ (cashew peduncle and produced water) and D) CAJAP (cashew peduncle and produced water), E) GLI and F)
|
GLIAP (glucose and water produced), All analyzes were performed in triplicate. Similar letters indicate that there was no significant statistical difference (p ≤ 0.05)
The results for GLI and GLIAP has indicated no significant changes in emulsion pHs when submitted to different temperatures and during observed period (Figures 5A and 5B). On the other hand, developed emulsions with CO and COAP have expressed a significant increasing of pH after 3 days of preparing emulsion, then emulsions was stored at 40°C. On seventh day, CO and COAP have showed a significant increased pH at 25°C and 40°C (Figures 5C and 5D). These results show that emulsions maintained pH stable over time at 4°C. However, pHs at other temperatures trend to be less stable with increasing temperature along time. Same behavior was observed in CAJ (Figure 5E). CAJAP did not change pH at observed temperatures (Figure 5F).
Stability of emulsions underwent great influence of pH. Studies have suggested that alkaline pH provides greater stability to emulsions.30 In O/W solutions, a higher pH may promote a huge affinity of surfactant molecules with their aggregations, which would result in a more stable solution.31 In this study, for different produced emulsions, pH changed from 6.0 to 8.0. However, as stated by some studies, higher temperatures may reduce viscosity of emulsions, eventually, destabilizing and breaking emulsions. Temperatures over range from 50 to 65°C may undermine completely emulsions.9,32 This mechanism may explain pH variation in emulsions, COAP, CAJ and CAJAP. In GLI and GLIAP emulsions, it was not noticed any difference of pH, which may be related to a more stable emulsion and less complex medium due to a more simple carbon source. On the other hand, in emulsions CO, COAP, CAJ and CAJAP there was variation of pH when the temperature was increased. This lower stability for these emulsions in relation to pH may be concerned to complexity of coconut and cashew biomasses, which have lignocellulosic components, proteins, phenolic components and others.33–35 These compounds may behave differently in emulsions when subjected to different and increasing temperatures, causing lower pH stability when compared to emulsions pre-filled using glucose (GLI) as carbon source or glucose and produced water like carbon source (GLIAP). Therefore, coconut and cashew residues can be used as a low cost of carbon source for rhamnolipid production by P. aeruginosa AP 029/GLVIIA. Moreover the reuse of produced water in oil well drilling as adjunct to culture media for rhamnolipid production can be performed.
Conclusion
This study found out that Moringa oleifera was residue among those studied which presented lower levels of cellulose and hemicellulose, and lower biosurfactant production. Coconut and cashew residues with and without presence of produced water in culture medium have displayed great capacity to maintain stability of emulsion through time. Coconut residue was detached due to its smaller variation of bubble size and greater reduction of surface tension of O/W emulsion, being inferior to that of emulsion with biosurfactant broth that used glucose as carbon source. Unlike behavior when using broths with glucose, the pH of O/W emulsions formed from coconut and cashew broth have presented variation in temperatures of 25°C and 40°C, being unchanged at 4°C. These outcomes confirm great potential of coconut and cashew residues for biosurfactant production as well as reducing costs of productive process, given high bioavailability of these residues.
Acknowledgments
The authors thank CAPES and Brazilian National Council for Research (CNPq) for financial support.
Conflicts of Interest
There is no conflict of interest.
References
- Das K, Mukherjee A.K. Differential utilization of pyrene as the sole source of carbon by Bacillus subtilis and Pseudomonas aeruginosa strains: Role of biosurfactants in enhancing bioavailability. J. Appl. Microbiol. 2007;102:195–203. doi:10.1111/j.1365-2672.2006.03070.x
CrossRef - Henkel M, Müller M.M, Kügler J.H, Lovaglio R.B, Contiero J, Syldatk C, Hausmann R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012;47:1207–1219 doi:10.1016/j.procbio.2012.04.018.
CrossRef - Bogaert V.I.N.A, Saerens K, Muynck D.C, Develter D, Soetaert W, Vandamme E.J. Microbial production and application of sophorolipids. 2007.
- Gudiña E.J, Rodrigues A.I, Freitas d.V, Azevedo Z, Teixeira J.A, Rodrigues L.R. Valorization of agro-industrial wastes towards the production of rhamnolipids. Bioresour. Technol. 2016;212:144–150. doi:10.1016/j.biortech. 2016.04.027.
CrossRef - Mnif I, Ghribi D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Biopolymers. 2015;104:129–147. doi:10.1002/bip.22630.
CrossRef - Pereira J.F.B, Gudiña E.J, Costa R, Vitorino R, Teixeira J.A, Coutinho J.A.P, Rodrigues L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel. 2013;111:259–268. doi:10.1016/j.fuel.2013.04.040.
CrossRef - Souza E.C, Vessoni-Penna T.C, Oliveira D.S.R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. 2014.
- Vaz D.A, Gudiña E.J, Alameda E.J, Teixeira J.A, Rodrigues L.R. Performance of a biosurfactant produced by a Bacillus subtilis strain isolated from crude oil samples as compared to commercial chemical surfactants. Colloids Surfaces B Biointerfaces. 2012;89:167–174. doi:10.1016/j.colsurfb.2011.09.009.
CrossRef - Varjani S.J, Upasani V.N. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour. Technol. 2017;232:389–397. doi:10.1016/j.biortech.2017.02.047.
CrossRef - Soberón-Chávez G, Lépine F, Déziel E. Production of rhamnolipids by Pseudomonas aeruginosa. 2005.
- Lai C.C, Huang Y.C, Wei Y.H, Chang J.S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009;167:609–614 . doi:10.1016/j.jhazmat. 2009.01.017.
CrossRef - Rufino R.D, de Luna J.M, de Campos T.G.M, Sarubbo L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014;17. doi:10.1016/j.ejbt. 2013.12.006.
CrossRef - Makkar R, Cameotra S. An update on the use of unconventional agro-industrial wastes for biosurfactant production and their new applications. 2002.
- Benincasa M, Contiero J, Manresa M.A, Moraes I.O. Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J. Food Eng. 2002;54:283–288. doi:10.1016/S0260-8774(01)00214-X.
- Radzuan M.N, Banat I.M, Winterburn J. Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour. Technol. 2017;225:99–105. doi:10.1016/j.biortech.2016.11.052
- Sluiter a, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C, Sluiter J, Templeton D, Nrel J.W. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. 2008;9. doi:NREL/TP-510-42621
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D. Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP). Nrel/Tp-510-42618. 2012;15. doi:NREL/TP-510-42618
- dos C , Mendes S, Oliveir A, da Bezerra S.M, de Padilha A.C.E, Melchuna A.M, de Macedo G.R, dos Santos E.S. Recovery of Rhamnolipids Produced by Pseudomonas aeruginosa Using Acidic Precipitation, Extraction, and Adsorption on Activated Carbon. Sep. Sci. Technol. 2013;48:2852–2859. doi:10.1080/01496395.2013.809107
- Peng X, Yuan X, Zeng G, Huang H, Zhong H. Extraction and purification of laccase by employing a novel rhamnolipid reversed micellar system. Process Biochem. 2012;47:742–748. doi:10.1016/j.procbio.2012.02.006
- Araujo C.K.C, de Campos O.A, Padilha A.C.E, de Junior S.F.C, do Nascimento R.J.A, de Macedo G.R, dos Santos E.S. Enhancing enzymatic hydrolysis of coconut husk through Pseudomonas aeruginosa AP 029/GLVIIA rhamnolipid preparation. 2016.
- Petrochemicals I. MATERIALS and METHODS. 1992;14:487–490.
- StaSoft Inc: StatSoft, http://www.statsoft.com/. 2005.
- Clien S.Y, Lu W.B, Wei Y.H, Chen W.M, Chang J.S. Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol. Prog. 2007;23:661–666. doi:10.1021/bp0700152
- Syldatk C, Lang S, Matulovic U, Wagner F. Production of four interfacial active rhamnolipids from ??-alkanes or glycerol by resting cells of pseudomonas species DSM 2874. Zeitschrift fur Naturforsch. – Sect. C .J. Biosci. 1985;40:61–67. doi:10.1515/znc-1985-1-213
- Agarwal T, Saxena M.K, Chandrawat M.P.S. Production and optimization of cellulase enzyme by Pseudomonas aeruginosa MTCC 4643 using sawdust as a substrate. Int. J. Sci. Res. Publ. 2014;4:4–6.
- Menéndez E, Ramírez-Bahena M.H, Fabryová A, Igual J.M, Benada O, Mateos P.F, Peix A, Kolařík M, García-Fraile P. Pseudomonas coleopterorum sp. nov., a cellulaseproducing bacterium isolated from the bark beetle Hylesinus fraxini. Int. J. Syst. Evol. Microbiol. 2015;65:2852–2858. doi:10.1099/ijs.0.000344.
- Arusha P.N, Kiran R.K, Shanti G.G, Arun S.K. Optimization of cellulase production for Bacillus sp. and Pseudomonas sp. soil isolates. African J. Microbiol. Res. 2016;10:410–419. doi:10.5897/AJMR2016.7954.
- Mulligan C.N. Environmental applications for biosurfactants. 2005.
- Lovaglio R.B, dos S.F.J, Jafelicci M, Contiero J. Rhamnolipid emulsifying activity and emulsion stability: PH rules. Colloids Surfaces B Biointerfaces. 2011;85:301–305. doi:10.1016/j.colsurfb. 2011.03.001.
- Devendiran D.K, Amirtham V.A. A review on preparation, characterization, properties and applications of nanofluids. 2016.
- Yang F, Niu Q, Lan Q, Sun D. Effect of dispersion pH on the formation and stability of Pickering emulsions stabilized by layered double hydroxides particles. J. Colloid Interface Sci. 2007;306:285–295. doi:10.1016/j.jcis.2006.10.062.
- Lim J.J.S, Law M.C.M, Samyudia Y, Dol S.S.S, Wong S, Law M.C.M, Samyudia Y, Dol, S.S.S. A Review on the Effects of Emulsions on Flow Behaviours and Common Factors Affecting the Stability of Emulsions. J. Appl. Sci. 2007;15:167–172. doi:10.3923/jas. 2015.167.172.
- Gonçalves F.A, Dos S.E.S, Macedo D.G.R. Use of cultivars of low cost, agro-industrial and urban waste in the production of cellulosic ethanol in Brazil: A proposal to utilization of microdistillery. 2015.
- da Silva F.L, de Campos O.A, dos S.D.A, de Júnior O.S.D, de Padilha A.C.E, de Junior S.F.C, de Macedo G.R, dos Santos E.S. Pretreatments of Carnauba (Copernicia prunifera) straw residue for production of cellulolytic enzymes by Trichorderma reesei CCT-2768 by solid state fermentation. Renew. Energy. 2018;116:299–308. doi:10.1016/j.renene.2017.09.064
- MacHado A.V, Oliveira E.L, Santos E.S, Oliveira J.A. Estudio del secado de anacardo (anacardium occidentale L.) mediante secador solar de radiación directa. Inf. Tecnol. 2010;21:31–37. doi:10.1612/inf.tecnol. 4137it.08.

This work is licensed under a Creative Commons Attribution 4.0 International License.





