Manuscript accepted on :
Published online on: --
Noorah Saleh Al-Sowayan
Department of Biology, Faculty of Science, Qassim University, P. O. Box: (30230), Buraydah (51744), Saudi Arabia. Corresponding Author E-mail: knaaj1@yahoo.com
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1031
ABSTRACT:
The purpose of this study was to examine the associations of serum concentrations of vitamin B6 and homocysteine with bone loss and fracture risk in postmenopausal female rats .Thus we try to visualize the effect ofovariectomy , estrogen replacement therapy and vitamin B6 supplementation on homocysteine levels and parameters of bone biomarkers. Adult female rats were classified as healthy control and ovariectomized control rats, the third group were ovariectomized rats received estrogen daily, the fifth and sixth group were ovariectomized rats received normal diet fortified with vitamin B6, and ovariectomized rats received estrogen daily with diet fortified with vitamin B6 respectivly. Fasting blood samples were measured for homocysteine, calcium ion, osteocalcin hormone, vitamin D3concentrations and alkaline phosphatase activity. The data from this study showed that indices of both bone resorption and formation increased markedly after ovariectomy.Serum homocysteine (Hcy) level ,vitamin. D3 and alkaline phosphatase activity were higher with insignificant changes in serum calcium and osteocalcin level in ovariectomized controlcompared with control healthy rats . Oral estrogen administration to ovariectomized rats induced higher concentration of serum Hcy when compared to normal control rats but Hcy concentration, alkaline phosphatase were decreased with insignificant decrease in serum Ca+2, vitamin D3 and increased osteocalcin level compared with ovatiectomized control group .Supplementation of vitamin.B6 alone to ovariectomized rats induced significant increase in osteocalcin accompanied with insignificant changes in serum Ca+2, and alkaline phosphates . VitaminD3 significantly increased in vitaminB6 treated rats than ovariectomized control rats.Combined treatment of ovariectomized rats with estrogen andvitamin B6 induced insignificant decrease in serum C+2 and return its level to normal value ,accompanied with significant increase in vitamin.D3 and osteaocalcin with decreased activity of alkaline phosphatase compared with withovariectomized group. The prevention of osteoporosis by identifying risk indicators as well as the development of new treatment strategies are major issues . Homocysteine is not only a risk factor ,but also a player in bone metabolism . However , this trial suggests that Hcy –lowering therapy may prevent bone loss in postmenopausal women .
KEYWORDS: Menopause; Estrogen deficiency; Bone disease, Rats.
| Copy the following to cite this article: Al-Sowayan N. S. Relation between Homocysteine and Vitamin B6 on Biochemical Bone Turnover Markers in Ovariectomized Rats. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Al-Sowayan N. S. Relation between Homocysteine and Vitamin B6 on Biochemical Bone Turnover Markers in Ovariectomized Rats. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9923 |
Introduction
Menopause related estrogen deficiency increase the risk of bone diseasesin postmenopausal women ( 1 ) . Also , the study of ( 2 ) showed the elevated levels of Hcy in the postmenopausal women of Burkina Faso must be viewed as a characteristic of older age and its metabolic consequence .
Elevation of Hcy is associated with an increased risk for bone fractures . Whether the risk is due to Hcy or at the reduced levels of cofactors necessary for its metabolism , such as folic acid or vitamin B is not completely clear (3 ) .
Recently the results of ( 4 ) demonstrate weak , but significant relations between Hcy and markers of organic and inorganic bone resorption , suggesting a mechanistic role of Hcy in bone metabolism . Hcy is thought to play an important role in the development of osteoporsis and fracture and methionine synthase reductase is an enzyme involved in the conversion of Hcy to methionine ,and certain genetic polymorphisms leading to reduced the enzyme activity may cause hyperhomocysteinemia (5) .
Exogenous hormones are used worldwide by more than 100 million women yearly as hormonal contraception or postmenopausal hormonal therapy ( 6 ) . Hormone replacement therapy ( HRT ) improves Hcy metabolism in postmenopausal women and this effect seems to be independent of vitamin status and may have positive implication for prevention of osteoporosis ( 7 ) .
Several studies investigate the effect of HRT on Hcy level and lipid peroxide level and suggest that estrogen alone or with medroxyprogesterone acetate as a HRT lower serum Hcy levels and lipid peroxide levels ( 8 , 9 ) .
Moreover , the study of ( 10 , 11 , 12 ) evaluate the effects of HRT with or without the addition of vitamin B supplementation on serum Hcy levels and their relationship to bone metabolism in post menopausal women and that Hcy levels significantly decreased in vitamin B6 treated group but HRT alone significantly increase Hcy level .
The study of ( 3) reported that lower serum vitamin B6 significant risk factor of osteoporosis with plasma Hcy having a lesser effect and vitamin B6 acting through Hcy may also have an effect on the skeleton albeit a weaker one than folic acid .
Material and Methods
In this work 50 mature female albino rats weighting 200±50 gm . The rats were housed under the prevailing atmospheric conditions allover the experimental period in the laboratory of physiology in Qassim University.
Experimental procedure
Ovariectomy of rats as described by ( 13 ) .
Two weeks after ovariectomy to ensure surgical menopause . The rats will divided into equal groups as follow :
Group I : Intact rats served as control ( I ).
Group II : Ovariectomized rats served as control ( II ) .
Group III : Ovariectomized rats received daily 2mg of estrogen orally ( 14) .
Group IV : Ovariectomized rats received normal diet fortified with vitamin. B6 in a dose of75mg/day (15)
Group V : Ovariectomized rats received daily dose of estrogen orally and maintained
on the diet fortified with vitamin B6in dose 75 mg /day .
Blood samples will obtained from the retrorbital sinus allover night fasted rats under light ether anesthesia with capillary tubes .
Blood immediately centrifuged and serum were collected and stored at -20˚c. until assayed for estimation of the following:
Homocysteine level .
Calcium ion concentration .
Vitamin D3 concentration .
Osteocalcin hormone concentration
Activity of alkaline phosphatase .
Results
The results of the present study revealed a highly significant increase in serum homocysteine level in ovariectomized rats, will oral estrogen administration to ovariectomized rats induced significant increase in serum Hcy in comparison to normal rats but when compared to ovatiectomized group a significant decrease occurs .Serumhomocysteine concentration significantly decrease in ovariectomized rats supplemented with vitamin B6 alone or combined with estrogen (table 1and figure 1).
The data from this study showed that indices of both bone resorption and formation increased markedly after ovariectomy. A significant increase in serum vitamin D3concentration and alkaline phosphatase activity with insignificant changes in serum calcium and osteocalcin in ovariectomized rats.
A significant decrease alkaline phosphatase accompanied with insignificant decrease in serum ca+2,vit. D3 and increased osteocalcin level in ovariectomized rats treated with estrogen. Supplementation of vitamin B6 alone to ovariectomized rats induced significant increase in osteocalcin,insignificant changes in serum ca+2, and alkaline phosphates activity, while vitamin D3 significantly increased in vitamin B6 treated rats than ovariectomized control rats, while combined treatment of ovariectomized rats with estrogen and vitamin B6 induced insignificant decrease in serum c+2 and return its level to normal ,accompanied with significant increase in vit.D3 and osteaocalcin with decreased activity of alkaline phosphatase (table 2 and figure 2).
Table 1: Effect of vitamin B6 administration on serum homocysteine levels)umol/L) in normal rats, Ovariectomized , Ovariectomized treated with estrogen .
| Parameter
Group |
Homocysteine | ||
| Mean±S.E. | (Ta) significant test | (Tb) significant test | |
| Control | 15.525±0.773 | – | 0.000* |
| Ovariectomized | 23.075±1.115 | 0.000* | – |
| Ovariectomized treated with estrogen | 19.412±0.364 | 0.004* | 0.006* |
| Ovariectomized treated with vitaminB6 | 17.625±1.074 | 0.104 | 0.000* |
| Ovariectomized treated with estrogen and vitaminB6 | 9.657±0.975 | 0.000* | 0.000* |
The mean difference is significant at the 0.05 level(*)
(Ta): significant as compared with normal control group.
(Tb):significant as compared with ovariectomized group.
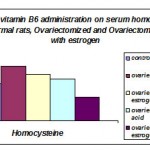 |
Figure 1
|
Table 2: Effect of vitamin B6 administration on serum calcium(mmol/L) , vitamin .D3, osteocalcin (ng/ml)and alkalin phosphatase activity(U/L) in normal rats, ovariectomized and ovariectomized treated with estrogen(ng/ml)
| Parameter
Group |
Calcium | Vitamen D3 | osteocalcin | Alkaline phosphatase | ||||||||
| Mean±S.E. | (Ta) significant test | (Tb) significant test | Mean±S.E. | (Ta) significant test | (Tb) significant test | Mean±S.E. | (Ta) significant test | (Tb) significant test | Mean±S.E. | (Ta) significant test | (Tb) significant test | |
| Control | 2.656±0.049 | – | 0.817 | 1.048±1.398 | – | 0.000* | 4.500±0.285 | – | 0.979 | 1.553±1.820 | – | 0.000 |
| Ovariectomized | 2.725±0.037 | 0.817 | – | 61.426±6.140 | 0.000* | – | 4.518±0.344 | 0.979 | – | 45.000±3.132 | 0.000 | – |
| Ovariectomized treated with estrogen | 3.144±0.477 | 0.123 | 0.170 | 54.401±4.536 | 0.000* | 0.377 | 3.841±0.227 | 0.332 | 0.303 | 80.714±12.444 | 0.000 | 0.005 |
| Ovariectomized treated with vitaminB6 | 2.580±0.048 | 0.807 | 0.629 | 93.530±4.945 | 0.162 | 0.000* | 7.912±0.715 | 0.000* | 0.000* | 67.833±13.250 | 0.000 | 0.069 |
| Ovariectomized treated with estrogen and vitaminB6 | 2.491±0.060 | 0.612 | 0.458 | 72.132±7.166 | 0.000* | 0.155 | 14.017±0.630 | 0.000* | 0.000* | 58.857±4.657 | 0.000 | 0.242 |
The mean difference is significant at the 0.05 level(*)
(Ta): significant as compared with normal control group.
(Tb):significant as compared with Ovariectomized group.
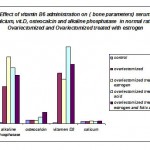 |
Figure 2
|
Discussion
In this study we induced hyperhomocysteinemia in female rats by bilateral ovariectomyto stimulate hyparhomocysteinemia induced by menopause.
The results of the present study revealed a highly significant increase in serum homocysteine in ovariectomized rats are in agreement with (16 and 17) whom reported a higher plasma homocysteine concentration in postmenopousal women rather than premenopausal women. Also, (18)contributed the higher level of total homocysteine in ovariectomizied rats to the alteration of the hormonal statud due to estrogen hormone deficiency.Elevatedhomocysteine is a strong risk factor for osteoporotic fractures among elderly , yet it may be a marker for low B vitamin status (19)
The effect of gonadal steroids on homocysteine is not yet full understood. Most studies reported on the effect of combined estrogen progesterone replacement therapy leaving the question of wether the estrogen or progestrogen component of this therapy was primarily responsible for the observed reduction of homocysteine (20).
In the present thesis administration of oral conjugated estrogen to ovariectomized rats resulting in a significant decrease in serum homocysteine level when compered to ovariectomized rats. This reduction are supported by the findings of (15&20) who found that oral estrogen as a replacement therapy in postmenopausal women reduced their high levels of plasma homocysteine which may contribute to the beneficial effect of estrogen replacement therapy on bone diseases (21 ).
The mechanism underlying the observed HRT induced decrease in homocysteine may be related to an increased kidney methionine synthetase, the regulating enzyme responsible for the remethylation of homocysteine to methionine (22). Also, hormone induced change in transamination of methionine could be a potential mechanism by which HRT can lower homocysteine concentration (23).
Supplementation of vitamin B6 on diet of ovariectomized rats resulting in a highly significant reduction in serum total homocysteine levels.This is suggested also by (24) who mentioned that B vitamin is required for remethylation of homocysteine to methionine which account for significant intracellular homocysteine consumption.
Vitamin B6 supplementation may determine a greater availability of methyl an sulphate groups as cofactor , substrates leading to a new synthesis of methionine but also to activation of other enzymatic pathways in the liver and kidneys (25) particularly, homocysteine and cys-Glycine in plasma interactvia redox and disulfide reactions becoming part of a dynamic system referred to as redox thiol status, which is linked to the antioxidants defence system (26).
Asignificant reduction in total homocysteine level observed inovariectomized rats cootreated with estrogen andvitamin B6 . These results are in agreement with (27) who evaluate the effect of postmenopausal oral HRT with or without folic acid supplementation on plasma homocysteine levels, they found that B vitamin and estradiol has a more potent lowering effect on plasma homocysteine level. Also, they suggested that co-treatment has a more prominent and significant effect on improving endothelial dysfunction than did estradiol alone.
As regard to the bone markers, the data from this study showed that indices of both bone resorption and formation increased markedly after ovariectomy. We observed a relationship between hyperhomocysteinemiaand bone marker of osteoporosis in ovariectomized rats. These may be attributed to loss of estrogens that accelerates effect of aging on bone by decreasing defense against oxidative stress (28).These may contribute to that homocysteine directly activates osteoclast formation and activity through increased generation of intracellular reactive oxygen species (29). Elevated Hcy is associated with reduced bone mineral density especially associated with bone fracture. The study of (30) suggested that HHcy and low folate associated with osteoporosis in postmenopausal women.Findings of (31) revealed high plasma Hcy induce apoptotic effect on osleoblasts.
The significant relations between Hcy and markers of organic and inorganic bone resorption, suggesting a mechanistic role of Hcy in bone metabolism. Also ,histological examination showed that an increased number of osteoclast cells with abnormal matrix in the cancellus and compact bone with cavity formation ovariectomized rats (32).
The insignificant increase in serum ca+2after ovariectomy may be due to enhancement of osteoclasts and increased number of mature osteoclasts, with continues release of calcium and phosphate as well as peptides from the bone matrix.
Our results are consistent with (33) who found a specific differences in calcium homeostasis in osteoporotic subjects.
A negative calcium balance must occur attributed to a primary process within the bone, with secondary consequences on the renal and gut handling of calcium or due to abnormalities in other organs and hormones regulating extra cellular calcium homeostasis, mainly PTH and osteocalcin hormone.
Also, PTH causes an increase of intestinal calcium absorption through its action on vit.D3, The active ca++ absorption in the duodenum is under the control of 1,25(OH2)D. This vit. D3 metabolite increase the intestinal cell synthesis of ca++ binding protein which enhances the net absorption of ca++. PTH also, decreases osteoblastic collagen synthesis, but osteoclastic bone resorption increases with a net increase of mineral release from bone into the extra cellular fluid (34).
Also, (35) reported that decreased estrogen level in female increased the sensitivity of bones to the action of PTH leading to bone resorption with lower bone mineral density . In contrast, serum ca++ level decreased in post menopausal women in the study of (36) .
Also, (37) have reported a decreased serum ca++ after ovariectomy in rats and the increased bone resorption does not appear to occur but the process of bone turnover increased.
The present work revealed significant increase in the serum vit. D3 and alkaline phosphatase with insignificant changes in osteocalcin level in ovariectomized rats. This results may be explained by the increased bone resorption due to an increase sensitivity of bone to PTH as a result of decreased plasma estrogen after ovariectomy and increased level of Hcy (38) .
Concerning, the increased alkaline phosphatase and vit. D3 after ovariectomy, indicates an increased osteoblastic activity on bone formation. In accordance (39) found a relationship between serum alkaline phosphatase and age, with marked rise after the first decade of menopause, also, (40) reported an increase in alkaline phosphatase activity after few days of ovariectomy.
The insignificant decrease in serum concentration of osteocalcin may be due to bone resorption after ovariectomy (41) and (42) found the same results after 9 weeks of ovariectomy. Also,(43) found that bone formation marker (osteocalcin) decreased in HHcy in rats due to bone resorption.
Inconssistent with our results(44) who found an elevation in ostecalcin concentration after 2 weeks of ovariectomy but its level decreased after 5 weeks and they explained these results by increased the process of bone formation.
Also,(45) were observed increased serum osteocalcin in premenopausal women and attributed that to increased bone loss before postmenopause as a result of increased ovulatory cycles.
The adverse effects of aging on the skeleton such as oxidative stress, are the fundamental mechanisms of the decline of bone mass and strength and osteocyte death is major contributor to the decline bone strength with age(46). Animals and humans studies suggest that homocysteine may weaken collagen crosslink’s and, if present in large amounts, interfere with bone remodeling (46).
The data of the present study showed a significant decrease in serum Hcy, alkaline phosphatase accompanied with insignificant decrease in serum ca+2, vit. D3 and increased osteocalcin level after estrogen treatment to ovariectomized rats .
This effects was explained by (47) that estrogen have a profound influence on Hcy and lipid peroxides and concluded that Hcy level controlled by administration of hormone replacement therapy .
From other hand, (48) reported that low serum vitamin B is a significant risk factor for osteoporosis, with plasma Hcy having a lesser effect and supplementation of folic acid may have an effect on the skeleton in postmenopausal women.
While (49) suggest that methionine synthase reductase (MTRR) is an enzyme involved in the conversion of Hcy to methionine and they hypothesized that certain genetic polymorphisms of MTRR leading to reduced enzyme activity may cause hyperhomocysteinemia and affect bone metabolism in postmenopausal women.
Also, the data of (50) suggest a major association between B vitamin and bone mineralization and high dietary, intake of B vitamin exerts positive effect on bone mineral density. Recent studies in rodents indicate that aging and the associated increase in reactive oxygen species (Ros) are the proximal culprits because it influence the generation and survival of osteoclasts, osteoblasts and osteocyte and loss of estrogen decreases defense against oxidative stress in bone and this accounts for increase bone resorption associated with loss of estrogen so, estrogen treatment may improve bone formation. In addition, increased glucocorticoid production and sensitivity with advancing age decrease skeletal hydration and thereby increase skeletal fragility by attenuating the volume of bone vasculature and interstitial fluid (51).
In this research supplementation of vit.B6 alone to ovariectomized rats induced significant increase in osteocalcin,insignificant changes in serum ca+2, and alkaline phosphates while vit.D3 significantly increased in vit.B6 treated rats than ovariectomized control rats. Coo- treatment of ovariectomized rats with estrogen and vitamin B6 induced insignificant decrease in serum c+2 and return its level to normal ,accompanied with significant increase in vit.D3 and osteaocalcin with decreased activity of alkaline phosphatase . So, combined treatment of rats with estrogen and vitamin B6 exhibit better action on bone turnover and prevents the loss of bone caused by estrogen deficiency in ovariectomized rats.
This data indicates an improvement in bone formation . This effects was explained by (52) that B vitamin act as coenzymes and show a close molecular interaction of the bases of the Hcy metabolism .The study of (19) suggested inversely association of mean bone loss and hip fracture risk in with vitamin B6 in elderly men and women . .Inconsistent with our results of ( 53)that elevated plasma homocysteine levels were found in adult and older female with eating disorders and there is not related to deficiencies in vitamin B .
The data of (23) are in agreement with our results, they reported a positive association between nutrient dense (intake of fruits, vegetable and whole grain) and bone health. Also, they suggest that B-vitamin supplementation to patients with HHcy may improve not only bone health, but also general health.
Also,(54) suggest that poor vitamin B6 has a synergistic effect on the risk of HHcy in elderly male and female.Disagrrement with our results ,the study of (55)on rats found that B vitamin deficiency induced HHcy but has no effect on bone health in healthy adult rats. Also, (5) suggested the association of vitamin B6 supplementation with Hcy reduction , but is unable to reduce the incidence of osteoporosis and fracture, becase in the majority of patients , Hcy is only moderetly increased .
So, there is a possibility that vitamin B6 could be added to estrogen therapy as a novel anti osteoporotic drug for menopausal women . Therefore, maintaining adequate B vitamin should be emphasized as an important measure for reducing Hcy level among elderly people, Also, we recommended that administration of estrogen and vitamin B6 has a beneficial effect to pre-and postmenopausal women to improve and prevent the occurrence of osteoporosis and fracture.
Acknowledgement
I wish to express my sincere gratitude and utmost thanks to the Dean of Scientific Research for funding this research .
References
- Sachdev, P. S. , M. J. Valenzuela , H. Brodaty , et al. , 2003 . Homocysteine as a risk factor for cognitive impairment in stroke patient . Dement Geriatr. Cogn. Disord. 15 ( 3 ) 155-162 . PMID : 12584431
- Gail, C. R. , P. A. Gail , B. B. Lynn , et al. , 2003 . Folate : A key to optimizing health and reducing disease risk in the elderly . Journal of the American college of Nutrition , 1-8 .
- Cagnacci, A. , M. Generali , D. Pirillo , F. Baldassari and A. Volpe , 2006 . Effects of low – or high – dose hormone therapy on fasting and post-methionine homocysteine levels in postmenopausal women . Climacteric. , 9 ( 5 ) : 388-395 . PMID : 17000586
- Ziakk, S. , G. Rammos , S. kountouris , et al. , 2001 . The effect of vitamin B6 and folate supplements on plasma homocysteine and lipids levels in patients on regular dialysis . Int. Urol. Nephrol. , 33 : 559-562 .
- Motivala, A.A. , P. A. Rose , H. M. Kim , Y. R. Smith , C. Bartnik , R. D. Brook , O. Muzik , C. S. Duvernoy , 2008 . Cardiovascular risk , obesity , and myocardial blood flow in postmenopausal women . J.Nucl. Cardiol. 15 ( 4 ):485-490 . PMID : 18674718
- Taechakraichana , N. , U. Jaisamrarn , K. Panykhamled , et al. , 2002 . Climacteric : concept , consequence and care . J. Med. Assoc. Thai.. 85 Sppl 1: S1-15 .PMID 12188398
- Ganong , W. F. , 2005 . Review of medical physiology .20ed . The gonads : Development and function of the reproductive system . Chapter 23 PP 411-453 .
- Moorthy , K. , U. C. Yadav , A. K. Mantha , S. M. Cowsik , D. Sharma , and N. Z. Baquer , 2004 . Estradial and progesterone treatment change the lipid profile in naturally menopausal rats from different age groups . Bioger. , 5 ( 6 ) : 411 – 419 .
- Sidibe, E. H. , 2005 . Menopause in Africa . Ann. Endocrinal . , ( paris ) 66 ( 2pti ) : 105-107 .PMID: 15959410.
- Barnabei, V. M. , D. Gardy , D. Stovall , et al. , 2002 . Menopausal symptoms in alder women and the effects of treatment with hormone therapy . Obstet. Gynecal. , 100 (6 ) : 1209-1218 .
- Magyar, Z. and T. Fel , 2006 . Treatment of menopausal symptoms . Review of the current literature. Orv. Hetil. 14 ; 147 ( 9 ) : 879-885 .
- Berg, G. , V. Meshch , L. Boero , F. Sayegh and H. Benencia , 2004 . Lipid and lipoprotein profile in menopausal transition effects of hormones , age and fat distribution . Horm. Metab. Res. 36 : 2150220 .
- Battezzati, A. , S. Bertoli , A. Romerio and G. Testolin , 2007 . Body composition : an important determinant of homocysteine and methionine concentrations in healthy individuals . Nutr. Metab. Cardiovasc. Dis. 17 ( 7 ) : 525-534 . PMID : 16860548 .
- EL-Swefy, S. , I.Sousou , A. Mervat and E. M. Hoda , 2002 . Hyperhomocysteinemia and cardiovascular risk in fenaleovariectomized rate ; role of folic acid and HRT . J. Pharm. Pharmacal. , 54 ( 3 ) : 391-397 .
- Sachdev, P. S. , M. J. Valenzuela , H. Brodaty , et al. , 2003 . Homocysteine as a risk factor for cognitive impairment in stroke patient . Dement Geriatr. Cogn. Disord. 15 ( 3 ) 155-162 . PMID : 12584431
- McFarlane, S. I. , R. Muniyappa , J. J. Shin , G. Bahtiyar and J. R. Sowers , 2004 . Osteoporosis and cardiovascular disease : brittle bones and boned arteries , is there a link ? Endocrine. , 23 ( 1 ) : 1-10 PMID : 15034190
- Bechmamn, G , 2001 . Physiologic aspects of natural and surgical menopause . J. Reprod. Med. , 46 ( 3 suppl ) : 307-315 .
- Karpral, A. J. Hyanek , J. Zivny , L. Dubska and T. Fait , 2002 . Homocysteinemia and ovariectomy initial experience with functional monitoring . Ceska. Gynekal. , 67 ( 6 ) : 328-332 .
- Lacey, J. , P. Mink , J. Lubin , M. Shermen , et al. , 2002 . Menopausal hormone replacement therapy and risk of ovarian cancer . Journal of the American Medical Association , 288 : 334-337 .
- Anderson, G. L. , M. Limacher , A. R. Assaf , et al. , 2004 . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy . Journal of the American Medical Association , 241 : 1701-1712 .
- Man, R. Y. , L. K. Ting , S. Fan , M. M. Lau , et al. , 2001 . Effect of postmenopausal hormone replacement therapy on lipoprotein and homocysteine levels in Chinese women . Climacteric. , 225 ( 1 ) : 129-`34 .
- Henry, D. , J. Roberston , D. O’connel and W. Grillespi , 1998 . A systematic review of the skeletal effects of estrogen therapy in postmenopausal women . Climacteric. , 1 : 92-111 .
- Rejnmark, L. , P. Vestergaard , A. P. Hermann , et al. , 2008 . Dictary intake of folate , but not vitamin B2 or B12 , is associated with increased bone mineral density 5 years after the menopause : result from a 10-year follow-up study in early postmenopausal women . Cacif. Tissue Int. , 82 ( 1 ) : 1-11 . PMID : 18175033
- Law, M. 2000 . Fortifying food with folic acid . Seminars in Thrombosis and Hemostasis . 26 ( 3 ) : 349-352 .
- Brattstrom, L. , D. E. L. Wilcken , J. Ohrvik and L. Brudin , 1998 . Common methylene tetrahyderofolatereductasegen mutation leads to hyperhomocysteinemia but not to vascular disease . The result of a metanalysus . circulation , 98 : 2520-2526 .
- Choi, S. W. and J. B. Mason , 2000 . Folate and carcinogenesis : An integrated scheme . J. Nutr. , 130 : 129-132 .
- Diaz Arrastia, R. , 2000 . Homocysteine and neurologic disease . Arch. Neural. , 57 : 1422-1427 .
- Paradisi, G. , F. Cucinelli , M. C. Mele , et al. , 2004 . Endothelial Function in post – menopausal women : effect of folic acid supplementation . Hum. Reprod. , 19 ( 4 ) : 1031-1035 . PMID : 15016776
- Creatsas, G. , G. Christodoulakos and I.Lambrinoudaki , 2005 . Cardiovascular disease : screening and management of the asymptomatic high – risk post-menopausal woman . Maturitas. , 52 ( Suppl 1 ) : S32-S37 . PMID : 16140482
- Chillemi, R. , J. Simpore , S. Persichilli , et al. , 2005 . Elevated levels of plasma homocysteine in postmenopausal women in Burkina Faso . Clin. Chem. Lab Med. , 43 ( 7 ) 765-771 . PMID : 16207140
- Baines, M. , M. B. Kredan , J. Usher , et al. , 2007 . The association of homocysteine and its determinants MTHFR genotype , folate , vitamin B12 and vitamin B6 with bone mineral density in postmenopausal British women . Bone. , 40 ( 3 ) : 730-736 . PMID : 17141597
- Herrmann, M. , M. Kraenzlin , G. Pape , et al. ( 2005) . Relation between homocysteine and biochemical bone turnover markers and bone mineral density in peri- and post- menopausal women . Clin. Chem. Lab Med. , 43 ( 10 ) 1118-1123 . PMID : 16197308
- Kim, D. J. , B. L. Park , J. M. Koh , et al. , 2006 . Methionine synthase reductase polymorphisms are associated with serum osteocalcin levels in postmenopausal women . Exp. Mol. Med. , 38 ( 5 ) : 519-524 . PMID : 17079868
- Marie, M. , H. L. Budev , et al. , 2003 . Hormone replacement therapy . Women’s health center at the Grau H women’s health and breast pavilion . 10 : 203 .
- Ventura, P. , A. Caganac , et al. , 2001 . Continous combined hormone replacement therapy with oral 17 beta-estradial and norethisterone acetate improves homocysteine metabolism in postmenopausal women . Menpoause , 8 ( 4 ) : 225-228 .
- Bednarek-Tupikowska, G. , K. Tupikowski , B. Bidzinska , et al. , 2005 . The effect of estrogen deficiency , estrogen and estroprogestagene therapy on total plasma homocysteine and serum lipid peroxide levels in postmenopausal women . Ginekol. Pol. , 76 ( 9 ) : 687-692 . PMID : 16417079
- – Burm, J. H. , D. J. Finney and L. G. Goodwin , 1952 . Bilateral oophorectomy , P. 241 : ” Biological standardization ” 2nd edition –Oxford University Press London and New York
- Gol, M. , P. Akan , E. Dogan , et al. , 2006 . Effect of estrogen , raloxifene , and hormone replacement therapy on serum C-reactive protein and homocysteine levels . Matturitas. , 53 ( 3 ) : 252-259 . PMID : 15990257 .
- Villa, P. , C. Perri , R. Suriano , et al. , 2005 . L-folic acid supplementation in healthy postmenopausal women : effect on homocysteine and glycolipid metabolism . J. Clin. Endocrinol. Metab. , 90 ( 8 ) : 4622-4629 . PMID : 15899950
- Toprak, A. , M. Erenus , A. H. Ilhan , et al. , 2005 . The effect of postmenopausal hormone therapy with or without folic acid supplementation on serum homocysteine level . Climacteric. , 8 ( 3 ) : 279- 286 . PMID : 16234277
- Hsu, S. C. , C. M. Liu , C. Y. Long , et al. , 2005 . Effect of oral conjugated equine estrogen combined with medroxyprogesterone acetate on plasma homocysteine levels in postmenopausal women . Fertil. Steril. , 84 ( 4 ) : 1037-1039 . PMID : 16213869
- 42- Gambacciani M. and P. Mannella (2007): Homocysteine, menopause and cardiovascular disease. Menopause Int 13(1): 23-6. PMID: 17448264
- 43- Castelao J.E. and M. Gago-Dominguez (2008): Risk factors for cardiovascular disease in women: relationship to lipid peroxidation and oxidative stress. Med Hypotheses. 71(1):39-44. PMID: 18308480
- 44- Bednarek-Tupikowska G. and K. Tupikowski (2004): Homocysteine–an underestimated atheromatosis risk factor. Do sex hormones influence homocysteine concentrations? PostepyHig Med Dosw (Online). 58:381-9.PMID: 15536396.
- 45- Perła-Kaján J., T. Twardowskiand H. Jakubowski (2007): Mechanisms of homocysteine toxicity in humans. Amino Acids. 32(4):561-72. Epub 2007 Feb 7.PMID: 17285228.
- 46- Rossi G.P., G. Maiolino, T.M. Seccia, A. Burlina, S. Zavattiero, M. Cesari, D. Sticchi, L. Pedon, M. Zanchettaand A.C. Pessina (2006): Hyperhomocysteinemia predicts total and cardiovascular mortality in high-risk women. J Hypertens. 24(5):851-9. PMID: 16612246.
- 47- Almeida M., L. Han, M. Martin-Millan, L.I. Plotkin, S.A. Stewart, P.K. Roberson, S. Kousteni, C.A. O’Brien, T. Bellido, A.M. Parfitt, R.S. Weinstein, R.L. Jilkaand S.C. Manolagas (2007): Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 282(37):27285-97. PMID: 17623659.
- 48- Papatheodorou L. and N. Weiss (2007): Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 9(11):1941-58.PMID: 17822365.
- 49- Glushchenko A.V. and D.W. Jacobsen (2007): Molecular targeting of proteins by L-homocysteine: mechanistic implications for vascular disease. Antioxid Redox Signal. 9(11):1883-98.PMID: 17760510.
- 50- Hachul de Campos H., L.C. Brandão, V. D’Almeida, B.H. Grego, L.R. Bittencourt, S. Tufik and E.C. Baracat (2006): Sleep disturbances, oxidative stress and cardiovascular risk parameters in postmenopausal women complaining of insomnia. Climacteric. 9(4):312-9.PMID: 16857662.
- 51 Ventura, P. , A. Caganac , et al. , 2001 . Continous combined hormone replacement therapy with oral 17 beta-estradial and norethisterone acetate improves homocysteine metabolism in postmenopausal women . Menpoause , 8 ( 4 ) : 225-228 .
- 52- Bonassi Machado R., E. ChadaBaracat, C. Eduardo Fernandes et al. (2007): Effect of estrogen and estrogen-progestogen therapy on homocysteine levels and their corrlation with carotid vascular resistance. Gynecol. Endocrinol. 23(11):619-24. PMID:17907004.
- 53- Chen K.J., W.H. Pan, F.L. Yang, I.L. Wei, N.S. Shaw and B.F Lin (2005): Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pac J ClinNutr. 14(3):250-5.PMID: 16169836.
- 54- Wolters M., A. Ströhleand A. Hahn (2004): Age-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequences. Z GerontolGeriatr. 37(2):109-35.PMID: 15103481.
- 55-Herrmann M., A. Tami, B. Wildemann, M. Wolny, A. Wagner, H. Schorr, O. Taban-Shomal, N. Umanskaya, S. Ross, P. Garcia, U. Hübner and W. Herrmann (2009): Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone. 44(3):467-75. PMID: 19056526.

This work is licensed under a Creative Commons Attribution 4.0 International License.





