Manuscript accepted on :
Published online on: --
Lantana camara; A Plant with Potential Anti-ulcerogenic Activity
Imran Kazmi1-2, Kamran Ahmad3, Shakir Saleem3, Muhammad Afzal1 and Firoz Anwar1
1Pacific University, Udaipur, Rajasthan, India.
2Siddhartha Institute of Pharmacy, Dehra Dun, Uttarakhand, India.
3Department of Pharmacology, Luqman College of Pharmacy, Gulbarga, Karnataka, India.
Corresponding author E-mail: afzalgufran@gmail.com
DOI : http://dx.doi.org/ http://dx.doi.org/10.13005/bbra/1058
ABSTRACT:
Lantana camara (Verbenaceae) is a medicinal plant traditionally used to treat various ailments, including gastrointestinal disorders, ulcers and internal sores. In order to establish pharmacological properties of the leaf of Lantana camara, studies were performed on antiulcer activity of the plant's aqueous and ethanolic extract. The Lantana camara aqueous extract (AELC) and ethanolic extract (EELC) was prepared in the doses of 250mg/kg and 500mg/kg. Antiulcer activity of AELC and EELC was evaluated by pyloric ligation and aspirin induced ulcer models. Acute toxicity was also carried out. The AELC and EELC dose dependently showed significant antisecretory activity as evidenced by decreased gastric juice volume, free acidity, total acidity, and increase in the pH of the gastric fluid in pylorus-ligated rats. Our studies also revealed that pretreatment with AELC and EELC significantly reduced the ulcer index in pylorus-ligated and aspirin treated rats in dose dependent manner. The antiulcer activity of Lantana camara is further supported by histopathological study which showed protection of mucosal layer from ulceration and inflammation. The present findings conclude that aqueous and ethanolic extract of Lantana camara has antiulcer effects; ethanolic extract is more potent then aqueous extract of Lantana camara and justify the traditional usage of this herb to treat ulcers.
KEYWORDS: Lantana camara; Antiulcer activity;Ulcer index; Aspirin; Pylorus ligation; Histopathology.
| Copy the following to cite this article: Kazmi I, Ahmad K, Saleem S, Afzal M, Anwar F. Lantana camara: A Plant with Potential Anti-ulcerogenic Activity. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Kazmi I, Ahmad K, Saleem S, Afzal M, Anwar F. Lantana camara: A Plant with Potential Anti-ulcerogenic Activity. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10074 |
Introduction
Plants have always a great importance in many cultures. Humans use them for their basic needs: feeding, clothing, sheltering, hunting and nursing. As source of medicines, plants have formed the basis for sophisticated traditional systems and continue providing mankind with new remedies. In recent years, the interest in folk medicine has highly increased. This discipline is gaining the scientific basis for its appropriate application within official medicine. The knowledge of herbal remedies, developed through trial and error over the centuries, is being used as guide to lead the chemists towards different classes of compounds. It is a fact that the 25% of all medical prescriptions are based on substances derived from plants or plant-derived synthetic analogues (Gurib-Fakim, 2006). Medicinal plants have traditionally occupied an important position in the socio-cultural, spiritual and health arena of rural and tribal lives of India. India has one of the oldest, richest and most diverse cultural traditions associated with the use of medicinal plants in the form of traditional systems of medicine (Government of India, 2000). The World Health Organization (WHO) estimates that 4 billion people, 80 percent of the world population, presently use herbal medicine for some aspect of primary health care (Farnsworth et al., 1985) and WHO notes that of 119 plant-derived pharmaceutical medicines, about 74 percent are used in modern medicine in ways that correlated directly with their traditional uses as plant medicines by native cultures (Ross, 2003). India is rich in cultural and floristic diversity and also a store house of ethno-botanical knowledge. Large sections of Indian population still rely on plant-based medicines as they are abundantly available, economical, and have little or no side-effects in addition to their cultural acceptability (Awaisu et al., 2010). The global demand for medicinal plants is increasing and, in India alone, the market is expanding at an annual rate of 20%, but only a little is known about its use-patterns (Uprety, 2010). For the past three decades, much information has been stored in the field of ethnobotany purely by qualitative overviews on human–plant relationship (Ragupathy and Newmaster, 2009).
Lantana camara Linn. is another Indian herbal plant, which has enormous traditional uses and used to treat lifestyle disorder (Kazmi et al., 2012). Lantana is mostly native to subtropical and tropical America, but a few taxa are indigenous to tropical Asia and Africa. It now occurs in approximately 50 countries where several species are cultivated under hundreds of cultivar names (Ghisalberti, 2000). The herb is in use since ancient times and recommended for the treatment of cancers, chicken pox, measles, asthma, ulcers, swellings, eczema, tumors, high blood pressure, bilious fevers, catarrhal infections, tetanus, rheumatism, malaria and ataxy of abdominal viscera (Kazmi et al., 2011). Different extracts plant has show anticonvulsant (Adesina, 1982), termicidal (Verma and Verma, 2006), wound healing (Nayak et al., 2009), anticancer (Bisi-Johnson et al., 2011), antiulcer, antioxidant (Sathish et al., 2011), anti-diabetic (Venkatachalam, et al., 2011), analgesic and anti-inflammatory (Ghosh et al., 2010), anti-motility (Sagar et al., 2005), anti-feedant, larval mortality/repellency (Pandey et al., 1983), antifungal and antibacterial (Barreto et al., 2010) activities.
Despite various claims on Lantana camara medicinal uses, particularly its potential to heal ulcer, no attempt has been made, to our best knowledge, to scientifically confirm on this matter. Thus, the aimed of the present study was to evaluate the antiulcer activity of Lantana camara leaf extract using various types of ulcerogenic models.
Materials and methods
Animals
Wistar albino male rats of 150-200 g were used for the study. The inbred colonies of rats were obtained from Siddhartha institute of Pharmacy, Dehradun (Uttarakhand). They were maintained in the animal house of Siddhartha institute of Pharmacy, Dehradun (Uttarakhand) for experimental purpose. The animals were maintained under controlled conditions of temperature (23 ± 2°C), humidity (50 ± 5%) and 12-h light-dark cycles. The animals were randomized into experimental and control groups and housed individually in sanitized polypropylene cages containing sterile paddy husk as bedding. They had free assessed to standard pellets as basal diet and water ad libitum. All the studies conducted were approved by the Institutional Animal Ethical Committee (IAEC) of Siddhartha institute of Pharmacy, Dehradun (Uttarakhand).
Collection of plant material
The leaves of Lantana camara were collected in the month of September 2010, from the Kumaun region, India and identified in Botany Department, Forest Research Institute, Dehradun. The voucher specimen (no.212) is deposited in the herbarium of Siddhartha Institute of Pharmacy, Dehradun (Uttarakhand)
Preparation of the extract
Dried powdered leaves of Lantana camara were grinded into small particles and soaked in distilled ethanol (90%) and distilled water (dH2O) in the ratio of 1:20 (w/v) to obtain the ethanol extract of Lantana camara (EELC) and aqueous extract of Lantana camara (AELC) and left for 24 h. Both mixtures were filtered using cloth filter, cotton wool and Whatman No. 1 filter paper to obtain the supernatant, which was subjected to freeze drying process. The extraction processes was repeated three times up to 72 h using the same residue. This method was carried out according to Zakaria et al., 2007b with slight modifications.
Acute (Oral) Toxicity Study (Fixed Dose Procedure)
Acute toxicity studies for ethanolic and aqueous extract of Lantana camara belonging to the family ‘Lamiaceae’ were conducted as per OECD guideline 420 using Albino Wister mice. Each animal was administered ethanolic and aqueous extracts solution by oral route. The test procedure minimizes the number of animals required to estimate the oral acute toxicity of a chemical and in addition estimation of LD50, confidence intervals. The test also allows the observation of signs of toxicity and can also be used to identify chemicals that are likely to have low toxicity (OECD guidelines, 2006).
As suggested, after acclimatization of animals for 4-5 days, study was carried out as follows:
Healthy, young adult Albino Swiss mice (20-25gms), nulliporous and non pregnant were used for this study food, but not water was with held for 3-4 hours and further 1-2 hours post administration of sample under study.
Fixed dose level of 5, 50, 500 mg/kg were initially chosen as dose level that would be expected to allow the identification of dose producing evident toxicity.
During the validation procedure, a fixed dose of 2000 mg/kg was added to provide more information on substance of low acute toxicity
Dosed one animal at the test dose by oral route. Since, this first test animal survived, four other animals were dosed (orally) as subsequent days, so that a total of five animals were tested.
Animals were observed individually at least every 5 minutes once during first 30 minutes after dosing, periodically at 2 hrs during the first 24 hours (with special attention during the first four hours) and daily thereafter, for a total of 14 days.
Anti-ulcer activity
Pylorus ligation induced gastric ulcers in rats
Albino wistar rats of either sex weighing between 150-200gms were divided into six groups of six animals each.
Group-I – Control.
Group-II – Standard (Ranitidine 20 mg/kg in 2% gum acacia p.o).
Group-III – Aqueous extract Lantana camara (250mg/kg p.o.).
Group-IV – Aqueous extract Lantana camara (500mg/kg p.o.).
Group-V – Ethanolic extract Lantana camara (250mg/kg p.o.).
Group-VI – Ethanolic extract Lantana camara (500mg/kg p.o.).
In this method albino rats were fasted in individual cages for 24 hr. care was taken to avoid coprophagy. Lantana camara leaf extracts or standard drug or control vehicle was administered 30 min. prior to pyloric ligation. Under light ether anesthesia, give an incision of 1cm long in the abdomen just below the sternum. Expose the stomach pass a thread around the pyloric sphincter and apply a tight knot. While putting the knot care was taken so that no blood vessels are tied along the knot. The abdomen was sutured clean the skin from any blood spots and bleeding. Apply collodion over the wound. At the end of 4 hr. after ligation the animals were sacrificed with excess of anesthetic ether. Open the abdomen and tie the oesophageal end (cardiac end) of the stomach. Cut and removed the entire stomach from the body of the animal. Gastric juice was collected into graduated centrifugation tube and was centrifuged at 1000 rpm for 10 min. and gastric volume was noted. The pH of the gastric juice was recorded by pH meter. Then the centrifuged supernatant contents were subjected to analysis for free and total acidity. Open the stomach along the greater curvature and washed with running water to see for ulcers in glandular portion of the stomach (Satoskar et al., 2005).
The number of ulcers per stomach was noted and severity of the ulcers scored microscopically with the help of hand lens (10X) and scoring was done as following.
0 = normal stomach, 0.5 = red coloration, 1.0 = spot ulcers, 1.5 = hemorrhagic streaks,
2.0 = ulcer > 3 but < 5, 3.0 = ulcer > 5
Mean ulcer score for each animal is expressed as ulcer index. The percentage protection was calculated using the formula,
Percentage protection = 100
Where, Ut = ulcer index of treated group.
Uc = ulcer index of control group.
Determination of free acidity and total acidity: –
One ml of gastric juice was pipeted into 100ml. conical flask, added 2 to 3 drops of topfer’s reagent and titrated with 0.01N NaOH until all traces of red color disappears and the color of the solution turns to yellowish orange. The volume of the alkali added was noted. This volume corresponds to free acidity. Then 2 to 3 drops of phenolphthalein solution was added and titration was continued until a define red tinge reappears. Again the total volume of alkali added was noted. The volume corresponds to total acidity. The results are incompiled in Table 3 and photographs of ulceration depicted in Fig. 1. Acidity was calculated by following formula:
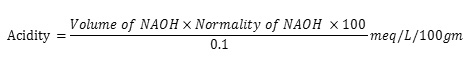
Table 1: Effect of aqueous and ethanolic extracts of Lantana camara leaves on gastric secretion following pyloric ligation in rats.
| Treatment Dose Vol. of | Free Total | PH |
| gastric juice acidity acidity | ||
| (ml) (mEq/L) (mEq/L) | ||
| 100gm 100gm | ||
| Control 2ml/kg 7.12±0.14 | 59.26±1.26 95.46±1.49 | 1.62±0.51 |
| (Distilled water)
|
||
| Ranitidine 20mg/kg 4.18±0.48c | 38.31±1.41c 46.04±1.24c | 4.68±0.20c |
| AELC 250mg/kg 6.63±1.01b | 52.84±0.69b 67.35±1.28b | 2.23±0.81b |
| AELC 500mg/kg 5.18±0.45c | 47.48±1.35c 53.50±1.24c | 3.39±0.56c |
| EELC 250mg/kg 6.38±1.10b | 56.30±1.49b 61.26±0.93b | 2.30±0.48c |
| EELC 500mg/kg 4.81±0.56c | 42.51±0.88c 51.74±1.05c | 4.43±0.76c |
Values are the mean S.E.M. of 6 rats / treatment
Significant *P < 0.05, **P < 0.01 and ***P < 0.001 compared with Control
Where as a=*P < 0.05, b=**P < 0.01 and c=***P < 0.001
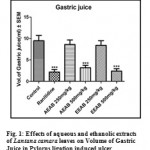 |
Figure 1
|
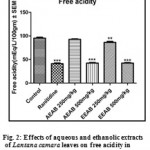 |
Figure 2
|
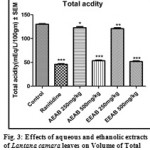 |
Figure 3
|
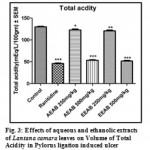 |
Figure 4
|
In this method following parameters was studied-
PH of gastric juice, Volume of gastric secretion, Free acidity, Total acidity, Ulcer index and
% Protection.
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on ulcer index and their % protection in pylorus ligation induced ulceration in rats
NSAID’s induced ulcer (Aspirin induced ulcer)
Albino wistar rats of either sex weighing between 150-200gms were divided into six groups of six animals each.
Group-I – Control (Aspirin 200mg/kg p.o.)
Group-II – Standard (Ranitidine 20 mg/kg in 2% gum acacia p.o).
Group-III – Aqueous extract Lantana camara (250mg/kg p.o.).
Group-IV – Aqueous extract Lantana camara (500mg/kg p.o.).
Group-V – Ethanolic extract Lantana camara (250mg/kg p.o.).
Group-VI – Ethanolic extract Lantana camara (500mg/kg p.o.).
On day 5, Aspirin at dose of 200mg/Kg was administered to the animals of all the groups (I to VI) one hour after the administration of last dose of the extract/ Ranitidine. Four hours after the administration of Aspirin, the animals were sacrificed and the stomach was then excised and cut along the greater curvature, washed carefully with 5.0 ml of 0.9% NaCl and ulcers were scored by a person unaware of the experimental protocol in the glandular portion of stomach. Ulcer index has been calculated by adding the total number of ulcers per stomach and the total severity of ulcers per stomach. The total severity of the ulcers was determined by recording the severity of each ulcer, and sample was send to further histopathological study (Satoskar et al., 2005).
The number of ulcers was noted and the severity recorded with the following scores:
0 = normal stomach, 0.5 = red coloration, 1.0 = spot ulcers, 1.5 = hemorrhagic streaks, 2.0 = ulcer > 3 but < 5, 3.0 = ulcer > 5.
Ulcer index and % Protection were calculated.
Histopathological evaluation
The stomachs were immersed in 10% formalin solution for histopathological examination. These tissues were processed and embedded in paraffin wax. The central part of damaged or ulcerated tissue (if present) was cut on half along the long diameter. If the stomach was protected from the damage then the section was taken from basal part using a rotary microtome, sections of thickness of about 5 μm were cut and stained with haemotoxylin and eosin. These were examined under the microscope for histopathological changes such as congestion, hemorrhage, necrosis, inflammation, infiltration, erosion and ulcer and photographs were taken.
Statistical analysis
Results were expressed as mean ± SEM, (n=6). Statistical analysis was performed with one way analysis of variance (ANOVA) followed by Tukey’s test using Prism 5 Graphpad software. P value less than <0.05 was considered to be statistically significant. *P<0.05, **P<0.01 and ***P<0.001, when compared with control and toxicant group as applicable.
Result
Preparation of extract
Successive Soxhlet extract process as yielded 3.1 % dark brown colored ethanolic extract and 3.5 % dark brown colored aqueous extract.
Acute toxicity (LD50) studies
There were no mortality and noticeable behavioral changes in all the groups tested. The extracts were found to be safe up to 2000 mg/kg body weight. An attempt was made to identify LD50 of ethanolic, aqueous extract of Lantana camara leaves. Since no mortality was observed at 2000 mg/kg. It was thought that 2000 mg/kg was the cut off dose. Therefore, 1/8 and 1/4 dose i.e. 250 mg/kg and 500 mg/kg were selected for all further in vivo studies.
Pylorus ligation ulcer model
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on volume of gastric secretion following pylorus ligation in rats
The volume of gastric juice secretion was significantly (P<0.001) reduced with a dose 500mg/kg of AELC and 500mg/kg of EELC in dose dependant manner when compared with control. The effect of Ranitidine (20mg/kg), AELC (250mg/kg and 500mg/kg) and EELC (250mg/kg and 500mg/kg) on volume of gastric juice secretion were 4.18, 6.63, 5.18, 6.38 and 4.81 ml respectively. The results are summarized in Table 1.
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on free acidity following pylorus ligation in rats.
The free acidity was significantly (P<0.01 and P<0.001) reduced with a dose of 500mg/kg of AELC, 250mg/kg and 500mg/kg of EELC in dose dependant manner when compared with control. The effect of Ranitidine (20mg/kg), AELC (250mg/kg and 500mg/kg) and EELC (250mg/kg and 500mg/kg) on free acidity were 38.31, 52.84, 47.48, 56.30 and 42.51 mEq/L respectively. The results are summarized in Table 1.
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on total acidity following pylorus ligation in rat
The total acidity was significantly (P<0.01 and P<0.001) reduced with a dose 250mg/kg and 500mg/kg of AELC and EELC in dose dependant manner when compared with control. The effect of Ranitidine (20mg/kg), AELC (250mg/kg and 500mg/kg) EELC and (250mg/kg and 500mg/kg) on total acidity was 46.04, 67.35, 53.50, 61.26 and 51.74 mEq/L respectively. The results are summarized in Table 1.
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on pH following pylorus ligation in rats
The pH was significantly (P<0.01 and P< 0.001) increased with a dose 500mg/kg of AELC and EELC in dose dependant manner when compared with control. The effect of Ranitidine (20mg/kg), AELC (250mg/kg and 500mg/kg), and EELC (250mg/kg and 500mg/kg) on pH were 4.68, 2.23, 3.39, 2.30 and 4.43 respectively. The results are summarized in Table 1.
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on Ulcer index and percentage protection following pylorus ligation in rats
Gastric lesions induced by pylorus ligation were significantly (P<0.01 and P<0.001) reduced by pretreatment with test extracts of 500mg/kg of AELC, 250mg/kg and 500mg/kg of EELC in dose related manner when compared with control. The reduction of ulcer index at a dose, 20mg/kg of Ranitidine, 250mg/kg and 500mg/kg of AELC and EELC were 2.01, 3.69, 2.54, 3.44 and 2.13 respectively. The percentage protection produced at a dose 20mg/kg of Ranitidine, 250mg/kg and 500mg/kg of AELC and EELC were 80.21%, 23.52%, 62.91%, 40.45% and 75.22% respectively. The ulcer protective action at 500 mg/kg dose of EELC was found to be closer to the reference standard drug, Ranitidine. The results are tabulated in Table 2.
Table 2: Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on ulcer index and their % protection in pylorus ligation induced ulceration in rats.
| Treatment | Dose | Ulcer Index | % Protection |
| Control | 2ml/kg | 4.82±0.89 | – |
| (Distilled water)
|
|||
| Ranitidine | 20mg/kg | 2.01±0.37c | 80.21 |
| AELC | 250mg/kg | 3.69±0.89b | 23.52 |
| AELC | 500mg/kg | 2.54±0.48c | 62.91 |
| EELC | 250mg/kg | 3.44±1.08b | 40.45 |
| EELC | 500mg/kg | 2.13±0.39c | 75.22 |
Values are the mean S.E.M. of 6 rats / treatment
Significant *P < 0.05 and ***P < 0.001 compared with Control
Where as a=*P < 0.05 and c=***P < 0.001
Aspirin induced ulcer
Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on ulcer index and their % protection in aspirin induced ulcer in rats
Gastric lesions induced by aspirin were significantly (P<0.05, P<0.01 and P<0.001) reduced by pretreatment with test extracts of 500mg/kg of AELC, 250mg/kg and 500mg/kg of EELC in dose related manner when compared with control. The reduction of ulcer index at a dose 20mg/kg of Ranitidine, 250mg/kg and 500mg/kg of AELC, and EELC were 1.01, 5.19, 2.26, 4.39 and 1.29 respectively. The percentage protection at a dose 20mg/kg of Ranitidine, 250mg/kg and 500mg/kg of AELC and EELC were 87.21%, 19.82%, 55.90%, 37.24% and 78.37% respectively. The ulcer protective action at 500 mg/kg dose of EELC was found to be closer to the reference standard drug, Ranitidine. The results are tabulated in Table 3 and the photograph of open stomach is affixed in Fig. 2.
Table 3: Effect of Aqueous and Ethanolic extracts of Lantana camara leaves on ulcer index and their % protection in aspirin induced ulcer in rats.
| Treatment | Dose | Ulcer Index | % Protection |
| Control | 2ml/kg | 5.27±1.24 | – |
| (Distilled water)
|
|||
| Ranitidine | 20mg/kg | 1.01±0.19c | 87.21 |
| AELC | 250mg/kg | 5.19±1.43a | 19.82 |
| AELC | 500mg/kg | 2.26±0.51c | 55.90 |
| EELC | 250mg/kg | 4.39±0.90b | 37.24 |
| EELC | 500mg/kg | 1.29±0.41c | 78.37 |
Values are the mean S.E.M. of 6 rats / treatment
Significant *P < 0.05, **P < 0.01 and ***P < 0.001 compared with Control
Where as a=*P < 0.05, b=**P < 0.01 and c=***P < 0.001
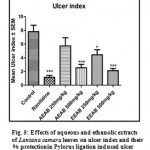 |
Figure 5
|
 |
Figure 6
|
Histopathological studies of aqueous and ethanolic extracts of Lantana camara leaves on Aspirin induced ulcer in rat.
In control group (Aspirin 200mg/kg) the mucosa showed redness, congestion, hemorrhagic sticks, inflammation, necrosis and dilation of blood vessels. In Ranitidine (20mg/kg p. o.) treated group, mucosa shows the mild redness, mild inflammation, and dilation of blood vessels. At 250 mg/kg p.o. of AELC treated group, the mucosa shows the redness, mild inflammation, congestion, hemorrhagic sticks and dilation of blood vessels. At 500 mg/kg p. o. of AELC treated group, the mucosa shows the mild redness, no inflammation, mild congestion, and mild dilation of blood vessels. At 250 mg/kg p. o. of EELC treated group, the mucosa shows the mild redness, mild inflammation, mild congestion and hemorrhage. At 500 mg/kg p. o. of EELC treated group, the mucosa shows the no dilation of blood vessels, no redness, no inflammation and mild congestion.The photographs of histopathological studies are affixed in Fig. 3.
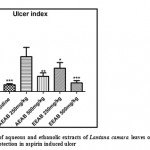 |
Figure 7
|
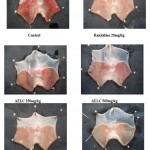 |
Figure 8
|
 |
Figure 9
|
Discussion
Gastric ulcer is one of the major gastrointestinal disorders, which occurs due to an imbalance between the offensive (gastric acid secretion) and defensive (gastric mucosal integrity) factors (Laine et al., 2008). Peptic ulcers are lesions in the gastrointestinal tract that usually occur in the stomach and duodenum and are characterized by mucosal damage resulting from the aggressive action of pepsin and gastric acid secretion. The disease affects millions of people around the world and is considered a major cause of morbidity and mortality worldwide. In the United States alone, approximately 500,000 individuals develop peptic ulcers each year (Ramakrishnan and Salinas, 2007). It is currently known that a number of factors are involved in the emergence of peptic ulcers, including Helicobacter pylori infection, chronic use of nonsteroidal anti-inflammatory drug (NSAID), the use of other drugs such as corticosteroids, bisphosphonates, anticoagulants and chemotherapy, gastric mucosa ischemia, age, genetic factors, a lifestyle involving stress, alcohol abuse, or smoking, and dietary habits (Stewart and Ackroyd, 2008).
Lantana camara is known to possess various therapeutic properties and has been one of the most noteworthy plants mentioned in various medicinal systems and also very common in folk medicines. In folk medicines it is used in gastrointestinal disorders, ulcer and internal sores (Kazmi et al., 2012). In our studies, we went a step ahead and have studied antiulcer activities of aqueous and ethanolic extract of Lantana camara leaves in two different models including pyloric ligation and aspirin induced ulcer model.
The causes of gastric ulcer in pyloric ligation are believed to be due to the imbalance between aggressive factors and mucosal integrity maintained by the indigenous defense mechanism (Mujumdar et al., 2003). The increased secretion of acid pepsin in pyloric ligation is reported and this may leads to auto digestion of gastric mucosa .In addition pyloric ligation may reduce GSH levels of gastric mucosa and increase the lipid peroxidation (Mahendran et al., 2002). There is one more report that pepsin is active in lower pH. Therefore the reduced gastric ulcer in this model may due to the reduction in acid secretion and increase gastric pH. These factors are associated with the development of upper gastrointestinal damage including lesions, ulcers and life threatening perforation and hemorrhage. In the present study, administration of Lantana camara leaves extract significantly decreased the gastric content, free acidity, total acidity, ulcer index and pH induced by pylorus ligature when compared to control. This may possibly be due to the antisecretory property of Lantana camara leaves extract.
When aspirin is in the lipid soluble undissociated form it can damage the gastric mucosa (Brown et al., 1977). Aspirin causes a dose dependent reduction in mucosal prostaglandins – PGE2 and PGI2 bio-synthesis accompanied by an increase in the mean area of gastric ulcerations. Aspirin is known to inactivate irreversibly the PG synthetase system, which mediates synthesis of prostaglandin in the mucosa (Vane, 1971). An increase in acid secretion and back diffusion at H+ ions, resulting in over production of leukotriens and other products of 5-lipoxygenase pathway. It is reasonable to assume that the observed gastric mucosal lesions induced by aspirin are due to a deficiency of mucosal prostaglandin (Obi et al., 2000). Aspirin induced ulcer is mediated through tissue damaging free radicals, which are produced from the conversion of hydroperoxyl to hydroxyl fatty acids, which leads to cell destruction. The hydroperoxyl fatty acids are generated from the degeneration of mast cells and generalized lipid per oxidation accompanying cell damage (Umamaheswari et al., 2007). Aspirin (200mg/kg) had shown severe ulceration as indicated by ulcer index i.e.7.27, whereas, pretreatment with a dose 500 mg/kg of AELC and 250mg/kg and 500 mg/kg of EELC has significantly (P<0.05, P<0.01 and P<0.001) reduced the ulcer index in a dose dependant manner when compared with control. It could be suggested that Lantana camara leaves extract can suppress gastric damage induced by aggressive factors. The antiulcer activity of Lantana camara leaves extract is further supported by histopathological study which showed protection of mucosal layer from ulceration and inflammation.
The present study demonstrated the potential of Lantana camara leaves to exert anti-ulcer activity especially the ethanolic extract. These findings, suggest that the observed antiulcer activity of Lantana camara leaves extract could be mediated partially through its anti-secretory and demulcent effect may be due to the presence of clerodane diterpenes, withaferin A and iridoid glycosides, which is already reported for anti-inflammatory activity or also may be due to the presence of secondary metabolites like alkaloids, flavonoids, steroids, triterpenoids, saponins and tannins like phenolic compounds which is reported for Hypoglycemic, Antihypertensive, Anti-inflammatory, Anticancer, Antibacterial, Immunomodulatory and Antispasmodic activity.
Conclusion
In conclusion, the present study demonstrated the potential of Lantana camara leaves extracts to exert anti-ulcer activity especially the ethanolic extract and thus justify the uses of the plant in treating ulcer related ailments.
References
- Adesina, S.K., 1982. Studies on some plants used as anticonvulsants in Amerindian and African traditional medicine. Fitoterapia 53:147–62. against brinjal aphid Aphis gossypii Glover. Indian J Ent 45:313–4.
- Awaisu, A., Samsudin, S., Amir, N.A., Omar, C.G., Hashim, M.I., Mohamad, M.H., Shafie, A.A., Hassali, M.A., 2010. Measurement of nicotine withdrawal symptoms: linguistic validation of the Wisconsin Smoking Withdrawal Scale (WSWS) in Malay. BMC Med Res Methodol 22(10), 46.
- Barreto, F., Sousa, E., Campos, A., Costa, J., Rodrigues, F., 2010. Antibacterial activity of Lantana camara Linn and Lantana montevidensis Brig extracts from Cariri-Ceará, Brazil. J Young Pharm 2:42–4.
- Bisi-Johnson, M.A., Obi, C.L., Hattori, T., Oshima, Y., Li, S., Kambizi, L., et al., 2011. Evaluation of the antibacterial and anticancer activities of some South African medicinal plants. BMC Complement Altern Med 11:14–8.
- Brown, B.K., Krause, W.J., Lvey, K.J., 1977. Effect of sodium bicarbonate on aspirin induced damage and potential deference changes in human gastric mucosa. Br Med J 2, 1052-5.
- Farnsworth, N.R., Akerele, O., Bingel, A.S., Soejarta, D.D., Eno, Z., 1985. Medicinal plants in therapy. Bull World Health Organ 63 (6), 965-981.
- Ghisalberti, E.L., 2000. Lantana camara L. (Verbenaceae). Fitoterapia 71: 467-486.
- Ghosh, S., Das Sarma, M., Patra, A., Hazra, B., 2010. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J Pharm Pharmacol 62:1158–66.
- Government of India, 2000. Report of the task force on conservation and sustainable use of medicinal plants. Planning commission, govt. India, New Delhi, India.
- Gurib-Fakim, A., 2006. Medicinal plants: traditions of yesterday and drugs of tomorrow.Molecular Aspects of Medicine 27, 1–93.
- Kazmi, I., Rahman, M., Afzal, M., Gupta, G., Saleem, S., Afzal, O., et al., 2012. Anti-diabetic potential of ursolic acid stearoyl glucoside: A new triterpenic glycosidic ester from Lantana camara. Fitoterapia 83:142–146.
- Laine, L, Takeuchi, K., Tarnawski, A., 2008. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 135, 41–60.
- Mahendran, P., Sabitha, K.E., Devi, C.V., 2002. Prevention of HCl-ethanol induced gastic mucosal injury in rats by Garcinia cambogia extract and its possible mechanism of action.J. Expl. Biol., 58-62.
- Mujumdar, B., Chaudhuri, S.G.R., Ray, A., Kumar, B.S., 2003. Effect of ethanol extract of Piper betle Linn. leaf on healing of NSAD’s induced experimental ulcer A novel role of free radical scavenging action. Indian J.Expl. Biol., 41, 311-15.
- Nayak, B.S., Raju, S.S., Eversley, M., Ramsubhag, A., 2009. Evaluation of wound healing activity of Lantana camara L. -a preclinical study. Phytother Res 23:241–5.
- Obi, E., Emeh, J.K., Orisakwe, O.E., Afonne, O.J., Ilondu, N.A., Agbasi, P.U., 2000. Investigation of the biochemical evidence for the antiulcerogenic activity of Synclisia scabrida Indian J Pharmacol. 32, 381-3.
- OECD guidelines for the testing of chemicals (Acute oral toxicity – Fixed dose procedure). Adopted 23rd march 2006. (cited 2008 Jun 20); Available from:URL:www.oecd.org
- Pandey, U.K., Srivastava, A., Lekha, C., Singh, A., 1983. Efficacy of certain plant extracts
- Ragupathy, S., Newmaster, S.G., 2009. Valorizing the ‘Irulas’ traditional knowledge of medicinal plants in Kodiakkarai Research Forest, India. Journal of Ethnobiology and Ethnomedicine 5, 10.
- Ramakrishnan, K., Salinas, R.C., 2007. Peptic ulcer disease. American Family Physician 76 1005–1012.
- Ross, I.A. 2003. Medicinal Plants Of The World, Volume 1: Chemical Constituents, Traditional And Modern Medicinal Uses, 2nd Ed., pp 26-34.
- Sagar, L., Sehgal, R., Ojha, S., 2005. Evaluation of antimotility effect of Lantana camara L. var. acuelata constituents on neostigmine induced gastrointestinal transit in mice. BMC Complement Altern Med 5:18–23.
- Sathish, R., Vyawahare, B., Natarajan, K., 2011. Antiulcerogenic activity of Lantana camara leaves on gastric and duodenal ulcers in experimental rats. J Ethnopharmacol 134:195–7.
- Satoskar, R.S., Bhandarkar, S.D., Nirmala, N.R., 2005. Pharmacology and Pharmacotherapeutics. Revised 19th edition. Mumbai Popular Prakashan Ltd., pp. 611- 25.
- Stewart, D.J., Ackroyd, R., 2008. Peptic ulcers and their complications. Surgery: Oesophagus & Stomach 26, 452–457.
- Umamaheswari, M., Ashokkumar, K., Rathidevi, R., Sivashanmugam, A.T., Subhadradevi, V., Ravi, T.K., 2007. Antiulcer and Invitro antioxidant activities of Jasminum grandiflorum L. J. Ethnopharmacology 110, 464-70.
- Uprety, Y., Asselin, H., Boon, E.K., Yadav, S., Shrestha, K.K., 2010. Indigenous use and bioefficacy of medicinal plants in the Rasuwa District, Central Nepal. Journal of Ethnobiology and Ethnomedicine 6, 3.
- Vane, J.R., 1971. Inhibition of prostaglandin synthesis as a mechanism of action of aspirin like drugs. Nature New Biol. 231, 232-5.
- Venkatachalam, T., Kumar, V.K., Selvi, P.K., Maske, A.O., Anbarasan, V., Kumar, P.S., 2011. Anti-diabetic activity of Lantana camara Linn fruits in normal and streptozotocin-induced diabetic rats. J Pharm Res 4:1550–2.
- Verma, R.K., Verma, S.K., 2006. Phytochemical and termiticidal study of Lantana camara var. aculeata leaves. Fitoterapia 77:46

This work is licensed under a Creative Commons Attribution 4.0 International License.





