Manuscript accepted on :
Published online on: --
Colin B. Lukong1*, Fortunatus Chidolue Ezebuo2 and Andrew W. Verla3
1Department of Biochemistry, Anambra State University, Uli, Nigeria.
2Department of Biochemistry, University of Nigeria, Nsukka, Nigeria.
3Department of Pure and Industrial Chemistry, Madonna University, Okija, Elele Campus, Nigeria.
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1035
ABSTRACT:
The gaint African snail Achatina achatina is farmed in many countries of West Africa and is much appreciated all over the world for its nutritional values. A. achatina employs aestivation to survive environmental stress such as lack of food and water. To assess some of the biochemical processes associated with aestivation, this study investigated the effect of prolonged aestivation on the composition of the major elements (K, Na, Ca, Mg, Cu, Fe and Zn) in the haemolymph of A. achatina using atomic absorption spectrophotometry. During the aestivation period, divalent elements (Ca, Mg) showed the highest increase followed by trace elements (Cu, Zn, Fe) and monovalent elements (Na, K). The increases were significant (p £ 0.01) for Na, Ca, Mg, Cu and Fe while K and Zn showed no significant (p £ 0.01) increase when compared between and within the weeks of aestivation. Increases in ion balance may have resulted from the need to compensate for the aestivation-induced decline in extracellular pH. The shell and some metallomacromolecules may have acted as a reservoir of ions that could be mobilized under extreme conditions hence making available minerals required for human health.
KEYWORDS: Giant African snails; aestivation;Achatina achatina; haemolymph; elements; nutrition
| Copy the following to cite this article: Lukong C. B, Ezebuo F. C, Verla A. W. Characterization of the Major Essential Elements in the Haemolymph of the Giant African Land Snail (Achatina achatina) during Aestivation. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Lukong C. B, Ezebuo F. C, Verla A. W. Characterization of the Major Essential Elements in the Haemolymph of the Giant African Land Snail (Achatina achatina) during Aestivation. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9941 |
Introduction
Mollusca are the second largest phylum of the animal kingdom, forming a major part of the world fauna. The Gastropoda is the only class of molluscs which have successfully invaded land. They are one of the most diverse groups of animals, both in shape and habit. They can be seen commonly in forest regions where rain fall is abundant (Odaibo, 1997; Robert, 2009). Among gastropods, land snails (subclass: Pulmonata) are one of the most numerous with the giant African snails (GAS), family Achatinidae being the largest species (Tillier, 1989). They are indigenous to Africa and are distributed in sub-Saharan Africa, ranging from the Gambia in the West to the Lake Chad region in the East. Their distribution extends southwards to the Orange River in South Africa (Hodasi 1995). The giant African Snails belong to two main genera namely Achatina (Lamarck) and Archachatina (Albers). The former occurs all over Africa whilst the latter is restricted to the West African sub-region. In Nigeria, Ghana, and other countries in West Africa, two species of the Achatina genus, namely Achatina achatina and Achatina fulica, and then two species of the Archachatina genus, i.e., Archachatina degneri and Archachatina marginata are known to occur (Monney, 1994).
The giant African snails respond to desiccating environmental conditions by entering aestivation, a behaviour which minimizes evaporative water loss (Hodasi, 1979; 1982). The giant African snails and other land snails have also evolved many morphological, physiological and biochemical adaptations to cope with hot and dry conditions (Umezurike and Iheanacho, 1983; Guppy et al., 1994; Storey, 2002). The period of aestivation, which may last up to several years, commences as the snail withdraws into its shell, secretes one or more epiphragm, and enters a metabolically quiescent condition (Hodasi, 1979). Rates of respiration may be reduced by more than 80% within days (Herreid, 1977; Pedler et al., 1996), and during extended aestivation oxygen consumption may approach undetectable levels (Schmidt-Nielsen et al., 1971). During aestivation, snails mobilize previously stored materials at much reduced rates which implies a reduction in food nutrients and an imminent weight loss (Umezurike and Iheanacho, 1983; Storey, 2002). Arousal from occurs in the wet seasons when the epiphragm breaks and the snail emerges to eat the new plant growth and the soft soil (Hodasi, 1979; 1982; Odaibo, 1997).
In most West and central African countries, such as Nigeria, Ghana and Cameroon, giant African snails are highly relished traditional food (Lubell, 2003; Avagnina, 2006). These snails are handpicked by the collectors and constitute a non-conventional protein source for the populace of west and central Africa. Popularly known as “Congo meat”, snail meat is a delicacy, cheap, easy to rear and could easily be a substitute for the expensive and diminishing conventional livestock protein obtained mainly from poultry, beef, mutton and pork (Oyenuga, 1968; Odaibo, 1997). Generally, snail meat is considered to be highly nutritious, owing to its content of essential amino acids, proteins, rich vitamins and minerals (Thanonkaew et al., 2006). Studies on the nutritional value of snail meat have reported that snail meat is high in proteins but low in fats hence an alternative food for people with high protein quality low fat diet requirements (Ademolu et al., 2004; Adeyeye and Afolabi, 2004; Fagbuaro et al., 2006; Babalola and Akinsoyinu, 2009; Çağıltay et al., 2011 ). Snail meat is low in cholesterol and high in health benefiting essential fatty acids such as linoleic acids and linolenic acids. This makes snail meat useful in the treatment of arteriosclerosis and other heart-related diseases (Imevbore and Ademosun, 1988; Fagbuaro et al., 2006; Abere and Lameed, 2008). Snails’ meat has also been associated with high levels of calcium, phosphorus, iron zinc and copper. Consumption of snails’ meat is therefore recommended for both old and young especially women who are in child-bearing age, pregnant and nursing women as this will combine effectively with other food components in providing the required essential elements to the body ( Babalola and Akinsoyinu, 2009). Due to its high iron content snail meat is also considered important in the treatment and prevention of anaemia while the shell particularly rich in calcium is very useful in the preparation of poultry feeds (Akinnusi, 1998; Okafor, 2001).
Great amount of research work refers to snail’s physiology as well as to their meat nutritive value; however not much information about the elemental content in their haemolymph especially during aestivation exists in the literature. The haemolymph is a very complex fluid in which the composition mirrors the physiological state of the organisms. The haemolymph composition was investigated with this aim in mind by comparing the concentrations of some essential minerals (Na, K, Mg, Ca, Cu, Zn, and Fe) in the haemolymph of one of the giant African snails; A. achatina during aestivation with those contained in the active animal and those obtained from snails’ meat. These elements have been examined before aestivation, during its onset, and through an aestivation period of 14 weeks.
In the present study we provide physiological strategies in relation to the extreme environment adaptations by this species. In a country like Nigeria, this study is extremely important, taking into account the high snail consumption by the Nigerian population. It is also relevant for the consumer, in general, to know the principal mineral characteristics of snails’ haemolymph which is most often discarded.
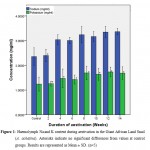 |
Figure 1: Haemolymph Na and K content during aestivation in the Giant African Land Snail (A. achatina). Asterisks indicate no significant differences from values at control groups. Results are represented as Mean ± SD. (n=5). |
Materials and Methods
Animals
Sixty snails (A. achatina) of weights ranging from 90 – 120 ± 10g were bought from commercial farmers along the Njaba River, Awomamma, Imo State, Nigeria and kept in oval palm frond made baskets of dimensions 75cm ´ 45cm ´ 35cm. The base of each basket was filled with soil (containing 85 mg CaCO3/ kg of soil) while its top was covered with wire mesh. Each basket had a total of ten snails and was humidified daily by sprinkling water inside. The animals were fed with fresh leaves of fluted pumpkin (Telferia occidentalis) and unripe pawpaw fruits for two weeks under laboratory conditions (25 – 270C). This time was allowed to ensure temperature acclimation and recovery from stresses caused by transportation and handling. At the end of the 2-week adjustment period, five snails were selected at random (the control group) and were deshelled to extract their haemolymph. Aestivation was induced in the remaining snails by the withdrawal of the daily supply of food and water. Under these conditions; room temperature (23-28°C) and humidity (ca. 20-60%), snails withdraw into their shells and quickly entered aestivation within 2 days. There was no indication that any of the animals became active again once they had entered quiescence. Photoperiod was not controlled. At the start of the experiment and at intervals of 2 weeks, following entry into aestivation, snails were sampled randomly from the baskets and deshelled to extract their haemolymph.
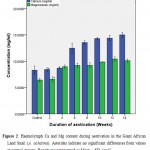 |
Figure 2: Haemolymph Ca and Mg content during aestivation in the Giant African Land Snail (A. achatina). Asterisks indicate no significant differences from values at control groups. Results are represented as Mean ± SD. (n=5). |
Collection of Haemolymph
Haemolymph samples were obtained by either capillary puncture using a glass pipette or a hypodermic needle (21gauge) above the pericardial region of the deshelled snail. Prior to puncturing, the sampling area was thoroughly dried with absorbent paper. Volumes of 20–30 ml of haemolymph were obtained from each individual.
The elements were measured by means of an atomic absorption spectrophotometer (Models SP 9, Pye Unican, UK). A minimum of two replicate analyses were performed for each sample. Concentrations were calculated from linear calibration plots obtained by measurement of the absorbance of standard solutions. All chemicals were of analytical grade obtained from Sigma, Germany and all reagents were freshly prepared unless otherwise stated.
 |
Figure 3: Haemolymph Cu, Zn and Fe content during aestivation in the Giant African Land Snail (A. achatina). Asterisks indicate no significant differences from values at control groups. Results are represented as Mean ± SD. (n=5). |
Statistical analyses
The data were expressed as Means ± Standard deviation (SD). Data analyses and presentation of graphs were performed using Pearson’s correlation and one way analysis of variance (ANOVA) with the statistical package for social science (SPSS) version 20 for windows. Significant differences were made at p ≤ 0.01 level.
Results
The concentrations of the major elements in the haemolymph of A. achatina are presented in Table 1. During the aestivation period, divalent elements (Ca, Mg) showed the highest increase. This was followed by trace elements (Cu, Zn, Fe) and monovalent elements (Na, K). The results showed significant (p ≤ 0.01) increase in the concentrations of sodium, calcium, magnesium, copper and iron while potassium and zinc showed no significant (p ≤ 0.01) increase when compared between and within the weeks of aestivation. . However, the increase in Na was significant relative to the control while that of K is not. Strong positive correlations (p ≤ 0.01) were observed between the concentrations of elements present in the haemolymph of the giant African snail during aestivation which suggest that, in 80% of the cases, the changes in the concentration of elements were directly proportional, and were most likely due to the effects of aestivation.
Table 1: Comparison of the major haemolymph elemental content during aestivation in the Giant African Land Snail (A. achatina).
| Duration of
aestivation (week) |
Mean Elemental content (mg/ml) | ||||||
| Na
|
K
|
Ca
|
Mg
|
Cu
|
Fe
|
Zn
|
|
| Control | 2.35667
± 0.355 |
1.24333
± 0.281 |
8.29667 ± 0.810 | 6.436667
± 0.348 |
1.063333
± 0. 123 |
2.280000
± 0.336 |
0.77333
± 0.050 |
| 2 | 2.40000
± 0.250* |
1.26000
± 0.100* |
8.43000
± 0.320* |
6.596667
± 0.435* |
1.0800
± 0.035* |
2.460000
± 0.180* |
0.833333
± 0.063* |
| 4 | 3.04000
± 0.203 |
1.47333
± 0.378* |
8.66667± 0.089* | 8.980000
± 0.318 |
2.1966
± 0.323 |
4.08666
± 0.272 |
1.160000
± 0.549* |
| 6 | 3.00667
± 0.125 |
1.42667
± 0.180* |
12.4633± 0.961 | 9.216667
± 0.410 |
2.3467
± 0.195 |
4.30000
± 0.150 |
1.28333
± 0.334* |
| 8 | 3.24000
± 0.329 |
1.693333
± 0.274* |
13.5433± 0.346 | 9.223333
± 0.502 |
2.5533
± 0.127 |
4.62000
± 0.436 |
1.2866667± 0.272* |
| 10 | 3.16667
± 0.196 |
1.64000
± 0.243* |
14.3100± 0.220 | 9.856667
± 0.046 |
2.4967
± 0.086 |
5.13333
± 0.194 |
1.2500
± 0.035* |
| 12 | 3.34667
± 0.229 |
1.73333
± 0.194* |
14.3900± 0.491 | 10.42667
± 0.411 |
2.7733
± 0.158 |
5.23333
± 0.076 |
1.286667
± 0.064* |
| 14 | 3.36333
± 0.125 |
1.686667
± 0.164* |
15.0033± 0.500 | 10.11667
± 0.297 |
2.7467
± 0.1069 |
5.37333
± 0.120 |
1.423333
± 0.442* |
Asterisks indicate no significant differences from values at control groups. Results are represented as Mean ± SD. (n=5)
The concentrations of monovalent elements (Na, K) present in the haemolymph of A. achatina during the 14 weeks of aestivation are presented in Figure 1. From these results Na showed the highest increase followed by K. Increases in the concentrations of Na were not significant (p ≤ 0.01) at week 2 but were significant (p ≤ 0.01) from weeks 4 to 14 when compared with the control. Meanwhile the concentrations of K did not increase significantly (p ≤ 0.01) from weeks 2 to 14 when compared with the control.
The concentrations of divalent elements (Ca, Mg) present in the haemolymph of A. achatina during the 14 weeks of aestivation are presented in Figure 2. The results showed that increases in calcium concentrations was not significant (p ≤ 0.01) at weeks 2 and 4 but increased significantly (p ≤ 0.01) from weeks 6 to 14 when compared with the control. Those of magnesium were not significant at week 2 but were significant (p ≤ 0.01) from weeks 4 to 14 when compared with the control.
The concentrations of trace elements (Cu, Zn, Fe) present in the haemolymph of A. achatina during the 14 weeks of aestivation are presented in Figure 3. Increases in the concentrations of copper and iron were not significant (p ≤ 0.01) at week 2 but significantly (p ≤ 0.01) increased from weeks 4 to 14 while increases in the concentrations of zinc were not significant (p ≤ 0.01) from weeks 2 to 14 when compared with the control.
Discussion
As shown from the results of the present study, aestivation induced different elemental changes in the haemolymph of the giant African snail and Na, Ca, Mg, Cu and Fe are the most clearly affected. In general as the duration of aestivation increases, elemental levels rose more suggesting that the ability to absorb water and excrete salts would become increasingly problematic causing internal ion disturbances. Other organisms exhibit similar patterns during aestivation or when faced with an environmental stress (Simkiss, 1983; Burton et al., 1987; Porcel et al., 1996; Lukong and Onwubiko, 2004b; Ong et al., 2004; Al-Azhary et al., 2008).
The results of the present investigation indicated increase of Na and K levels as aestivation prolongs. These results are not surprising since K and Na are essential to many physical functions. K and Na tend to accumulate in the organisms, keeping the osmotic pressure and retaining the water in the cells (Simkiss, 1983). It is known that the Na+-K+-ATPase activity in land snails is impacted by aestivation where there is a significant decrease of the Na+-K+-ATPase activity during acclimation to aestivation (Ramnanan and Storey, 2006). It has been suggested that Na+-K+-ATPase is of central importance in ion regulation in land snails and is responsible for the transport of Na+ with K+ from the cell into the haemolymph (Giokas et al., 2005; Ramnanan and Storey, 2006) which suggest that a disturbance in these ions might occur.
The concentrations of divalent ions especially that of Ca increased significantly during aestivation. Ca and Mg are responsible for nerve transmission, muscle contraction, renal functions and respiration. Calcium and Mg ions have the ubiquitous role as intracellular messengers, modulating the oxygen affinity of haemocyanin, transducing electrical and hormonal signals (Burton, 1976; Simkiss, 1983; Lukong and Onwubiko, 2004a; b). Since calcium is an important constituent of shells and many other rigid mechanical structures, the nerve of snails received excessive level of calcium making them move slowly. This explains the limited movement found in snails during aestivation. Though the overall balance of ion concentration in the haemolymph is important, the excess of calcium content and the increase in magnesium in snails may function to modulate the oxygen affinity of hemocyanin (Lukong and Onwubiko, 2004a; b). The elevated divalent ions in this study as aestivation prolongs may also function to offset metabolic and respiratory acidosis by buffering the extracellular fluid and hemolymph. The mobilisation of an internal source of buffer base (CaCO3 from the shell) is responsible for the elevated haemolymph Ca and Mg levels as described in Helix aspersa by Porcel et al., (1996). It is also probable that the animals in this study became dehydrated (water loss) resulting in elevated haemolymph ion concentrations including calcium.
Haemolymph trace metal concentrations show broad variations in relation to aestivation. This result is in accordance with the finding that trace metal content in pulmonate gastropods is regulated within a narrow range (Marigómez et al., 2002; Dallinger et al., 2005; Manso et al., 2007). Trace metals are commonly bound to proteins, and involved in numerous enzyme systems as cofactors (Harton and Perona, 2001). Concerning Cu, its changes are even more significant considering its involvement in haemocyanin as the respiratory pigment of snails (van Holde and Miller, 1995; van Holde et al., 2001). The zinc concentrations in this study did not change significantly throughout the experimental procedure while the concentrations of Fe showed significant changes. Iron is a constituent of several intracellular enzyme systems, notably the cytochromes (Harton and Perona, 2001). Although the total amount of iron in organism is not really much, the excess iron in haemolymph of aestivating snails can possibly hinder the activities of enzymes.
The presence of Fe, Cu and Zn in high concentrations in the haemolymph during aestivation is somehow expected, as these metals may be required as metal cofactors in the activation of various types of superoxide dismutase (Bowler et al., 1992; Brouwer et al., 2003; Culotta et al., 2006) which constitutes the first line of defense against oxidative damage. This adjustment to antioxidant defense would minimize damage due to a burst of reactive oxygen species generation, occurring as a result of a rapid increase in oxygen consumption over the early minutes of arousal from aestivation (Hermes-Lima et al., 1998; 2001). Also to sustain continuous aestivation for over a year or more, good antioxidant defenses in snails are critical to preventing or minimizing the accumulation of oxyradical damage products during prolonged aestivation (Storey, 2002).
Nutritionally, our study shows that the haemolymph of aestivating A. achatina has higher concentrations of minerals when compared to snail meat (Fagbuaro et al., 2006; Babalola and Akinsoyinu, 2009; Çağıltay et al., 2011). Nowadays in Nigeria, the highly nutritious snail meat has high economic value and is considered to be a luxurious traditional food. As a result snail meat together with the haemolymph serves as a significant and essential part of their daily meals and constitutes the major and cheapest source of minerals and other essential nutrients (Oyenuga, 1968; Odaibo, 1997). Of much concern are the high concentrations of Fe, Cu, and Zn which are also essential for all life forms but when present above the daily dose recommended by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) might become toxic to the human organism.
Conclusion
Aestivation stress induced changes in the haemolymph elemental content of the giant African snail; A. achatina. These probably contributed to the physiological changes which are some of the specific biochemical adaptations which enable snails to survive long term aestivation. The diligent collection and inclusion of snails’ haemolymph into the various traditional preparations is considered to be of high importance as its consumption will definitely increase the level of some major elements in the body.
References
- Abere, S.A. and Lameed, G.A. (2008). The medicinal utilization of snails in some selected states in Nigeria. In: Proceeding of the first National conference of the Forests and Forest Products Society (FFPs). Onyekwelu, J.C., Adekunle, V.A.J. and Oke, D.O. (eds.).Held in Akure, Ondo State between 11th and 18th of April, 2008. pp 233 – 237.
- Ademolu, K.O., Idowu, A.B., Mafiana, C.F. and Osinowo, O.A. (2004). Performance, proximate and mineral analyses of African giant land snail (Archachatina marginata) fed different nitrogen sources. African Journal of Biotechnology; 3(8): 412-417.
- Adeyeye, E.I. and Afolabi, E.O. (2004). Amino acid composition of three different types of land snails consumed in Nigeria. Food Chemistry; 85: 535-539.
- Akinnusi, O. (1998). A practical approach to backyard snail farming. Nigeria Journal of Animal Production; 25: 85-95.
- Al-Azhary D, B., Tawfek, N. S., Meligi, N. M. and Elliott, M. (2008). Physiological Responses to Hyper-Saline Waters in Necora puber (Velvet Crab). Pakistan Journal Physiology; 4(2): 1-6.
- Avagnina, G., (2006). Snail Breeding. Intensive Snail Breeding Complete Production Cycle Trading. Cherasco, Italy.
- Babalola, O.O. and Akinsoyinu, A.O.(2009). Proximate composition and mineral profile of snail meat from different breeds of land snail in Nigeria. Pakistan Journal of Nutrition; 8 (12): 1842-1844.
- Bowler, C., Montagu, M.V. and Inzé, D. (1992). Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology: Plant Molecular Biology; 43: 83–116.
- Brouwer, M., Hoexum Brouwer, T., Grater,W. and Brown-Peterson, N.(2003). Replacement of a cytosolic copper/zinc superoxide dismutase by a novel cytosolic manganese superoxide dismutase in crustaceans that use copper (haemocyanin) for oxygen transport. Biochemical Journal; 374: 219–228.
- Burton, R.F, Shirley, M. and Douglas, A. M. (1987). Some effects of injected MgCl2 in the snail Helix aspersa. Narcosis, magnesium distribution and responses in infused potassium. Comparative Biochemistry and Physiology; 86A: 113-117.
- Burton, R.F. (1976). Ca metabolism and acid-base balance in Helix pomatia. In: Perspectives of Experimental Biology- Zoology, Davies, P. S. (ed), vol. 1, Pergamon Press, Oxford, pp 7–16.
- Çağıltay, F., Erkan, N., Tosun, D. and Selçuk, A. (2011). Amino acid, fatty acid, vitamin and mineral contents of the edible garden snail (Helix aspersa). Journal of Fisheries Sciences; 5(4): 354-363.
- Culotta, V.C., Yang, M. and O’Halloran, T.V. (2006). Activation of superoxide dismutases: putting the metal to the pedal. Biochimica Biophysica Acta; 1763 (7): 747–758.
- Dallinger, R., Chabicovsky, M., Hödl, E., Prem, C., Hunziker, P. and Manzl, C. (2005).Copper in Helix pomatia (Gastropoda) is regulated by one single cell type: differently responsive metal pools in rhogocytes. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 289(4): R1185-R1195.
- Fagbuaro, O., Oso, J.A., Edward, J.B. and Ogunleye, R.F.(2006). Nutritional status of four species of giant land snails in Nigeria. Journal of Zhejiang University Science B; 7(9):686-689.
- Giokas, S., Pafilis, P. and Valakos, E. (2005). Ecological and physiological adaptations of the land snail Albinaria caerulea (Gastropoda, Pulmonata, Clausiliidae). Journal of Molluscan Studies; 71: 15–23.
- Harton, N.C. and Perona, J.J. (2001). Making the most of metal ions. Nature structural Biology; 8: 290 – 293.
- Hermes-Lima, M. and Storey, K.B. (1995). Antioxidant defenses and metabolic depression in a pulmonate land snail. American Journal of Physiology; 268: R1386–R1393.
- Hermes-Lima, M., Storey, J.M. and Storey, K.B. (1998). Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comparative Biochemistry and Physiology; 120B: 437–448.
- Hermes-Lima, M., Storey, J.M. and Storey, K.B. (2001). Antioxidant defenses and animal adaptation to oxygen availability during environmental stress. In: Cell and Molecular Responses to Stress—Protein Adaptations and Signal Transduction, Storey, K.B. and Storey, J.M. (Eds.), Vol. 2. Elsevier Press, Amsterdam, pp. 263–287.
- Hermes-Lima, M., Storey, J.M., Storey, K.B. (1998). Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comparative Biochemistry and Physiology; 120B:437–448.
- Herreid, C.F. (1977). Metabolism of land snails (Otala lactea) during dormancy, arousal and activity. Comparative Biochemistry and Physiology; 56A: 211–215.
- Hodasi J. K. M. (1995) Snails in the National Economy. Inaugural lecture delivered at the University of Ghana on 9th May 1991. Ghana Universities Press. pp 41.
- Hodasi J.K.M (1982). The effects of different light regimes on the behavior and biology of Achatina (achatina) achatina (Linne). Journal of Molluscan Studies; 48: 283 – 293.
- Hodasi, J.K.M. (1979). Life History of Achatina achatina (Linne). Journal of Molluscan Studies; 45: 328-339.
- Lubell, D. (2003). Are land snails a signature for the Mesolithic-Neolithic transition? 10th Neolithic Seminar the Neolithization of Eurasia – Paradigms, Models and Concepts Involved Ljubljana, Thursday 6th – Saturday 8th November 2003. 24 pages.http://www.manandmollusc.net/Lubell_Ljubljana1.pdf.
- Lukong, C. B. and Onwubiko, H.A. (2004b). Calcium, copper, protein and oxygen affinity of haemocyanin from aestivating and nonaestivating snails (Achatina achatina). Bio-research; 2(1): 115-123.
- Lukong, C.B. and Onwubiko, H.A. (2004a). A role for Ca2+ in the thermal and urea denaturation of haemocyanin from aestivating giant African snails Achatina achatina. Bio-Research; 2(2): 54-62.
- Manso, M., Carvalho, M. L. and Nunes, M. L. (2007). Characterization of essential and toxic elements in cephalopod tissues by EDXRF and AAS. X-Ray Spectrometry; 36: 413–418.
- Marigómez, I., Soto, M., Cajaraville, M.P., Angulo, E. and Giamberini L. (2002). Cellular and subcellular distribution of metals in molluscs. Microscope Research Technology; 56: 358–392.
- Monney K.A. (1994) Notable notes on giant African snails. Snail Farming Research. Vol. V: Associazione Nationale Elicicoltori (The Italian Snail Farmers Association), pp 1 – 22.
- Odaibo, A.B. (1997). Snail and Snail Farming. Nigeria Edible Land Snails. Stirling-Horden Publishers, Ibadan, Vol. 1, pp 1-11.
- Okafor, F. C. (2001). Edible land snail: A Manual of Breeding Management of Achatima achatina. Simarch Publizahas, Lagos pp 72.
- Ong, J.H.L., Chejlava, M., Fried, B., Koehnlein, K.M., Bosavage, G.L.and Sherma, J. (2004). Effects of Schistosoma mansoni infection on inorganic elements in the snail Biomphalaria glabrata. Journal of Helminthology; 78:343–346.
- Oyenuga, V.A. (1968). Agriculture in Nigeria. Rome, FAO.
- Pedler, S., Fuery, C.J., Withers, P.C., Flanigan, J. and Guppy, M. (1996). Effectors of metabolic depression in an estivating pulmonate snail (Helix aspersa): whole animal and in vitro tissue studies. Journal of Comparative Physiology; 166B: 375–381.
- Porcel, D., Bueno, J. D., and Almendros, A. (1996) Alterations in the digestive gland and shell of the snail Helix aspersa (Gastropoda, Pulmonata) after prolonged starvation. Comparative Biochemistry and Physiology; 115A: 11–17.
- Ramnanan, C.J., Storey, K.B. (2006). Suppression of Na+K+-ATPase activity during aestivation in the land snail Otala lactea. Journal of Experimental Biology; 209:677–688.
- Ramos-Vasconcelos, G.R. and Hermes-Lima, M. (2003). Hypometabolism, antioxidant defenses and free radical metabolism in the pulmonate land snail Helix aspersa. Journal of Experimental Biology; 206:675–685.
- Robert, N. (2009). Amazing facts about snails. Snails and Slugs (Gastropoda), Vienna, Austria pp 1999–2009.
- Schmidt-Nielsen, K., Taylor, C. R. And Shkolnik, A. (1971). Desert snails: problems of heat, water and food. Journal of Experimental Biology; 55: 385-398.
- Simkiss, K. and Mason, A.Z.(1983) Metal ions: metabolic and toxic effects. In: The Mollusca. Environmental Biochemistry and Physiology, Hochachka, P.W. (ed) vol. 2, New York Academic, pp. 102–164.
- Storey, K. B. (2002). Life in the slow lane: molecular mechanisms of aestivation Comparative Biochemistry and Physiology; 133A: 733–754.
- Storey, K.B. (2002). Life in the low lane: Molecular mechanism of aestivation. Comparative Biochemistry and Physiology; 133A: 733 – 754.
- Thanonkaew, A., Benjakul, S. and Visessanguan, W. (2006). Chemical composition and thermal property of cuttlefish (Sepia pharaonis) muscle. Journal of Food Composition and Analysis; 19: 127–133.
- Tillier, S. (1989). Comparative morphology, phylogeny and classification of land snails and slugs (Gastropoda, pulmonata, stylommatophosa). Malacologia; 30: 1 – 304.
- Umezurike, G.M. and Iheanacho, E.N. (1983). Metabolic adaptations in aestivating giant African snail (Achatina achatina). Comparative Biochemistry and Physiology; 74B: 493-498.
- Umezurike, G.M. and Iheanacho, E.N. (1983). Metabolic adaptations in aestivating giant African snail (Achatina achatina). Comparative Biochemistry and Physiology; 74B: 493-498.
- van Holde, K. and Miller, K. (1995). Hemocyanins. Advances in Protein Chemistry; 47: 1–81.
- van Holde, K., Miller, K. and Decker, H. (2001). Hemocyanins and invertebrate evolution. Journal of Biological Chemistry; 276: 15563–15566.

This work is licensed under a Creative Commons Attribution 4.0 International License.





