Manuscript accepted on : 15 May 2012
Published online on: --
T. C. Ozoko1 and A. O. Egwunyenga2
1Department Of Medical Microbiology and Parasitology, Delta State University Abraka, Delta State, Nigeria. 2Department Of Animal and Environmental Biology, Delta State University Abraka. Delta State, Nigeria.
ABSTRACT: This study aims to provide baseline data of the indices of malaria prevalence in the study population as well as other malariometric indices namely use of mosquito nets, subjective observation of mosquito bite nodules, parasite density. The objectives include an assessment of frequency of use of mosquito-net among students of Delta State University, Abraka using the study sample. Frequency of observation of mosquito bite nodules among the volunteers, frequency of useof anti-malarial medication in the last three months and malaria parasite density associated with level of use of mosquito nets. This study will help assess the impact of efforts to roll back malaria in the study population.
KEYWORDS: Mosquito Bite; Malarial Parasite; Abraka; Nigeria
Download this article as:| Copy the following to cite this article: Ozoko T. C, Egwunyenga A. O. Volunteer Perception of Mosquito Bite Nodules and Malaria Parasite Density in Abraka, Southern Nigeria. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Ozoko T. C, Egwunyenga A. O. Volunteer Perception of Mosquito Bite Nodules and Malaria Parasite Density in Abraka, Southern Nigeria. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9473 |
Introduction
Malaria parasite density is also called parasite count and is a malariometric index which focuses on assessing the severity of the burden of malaria infection (Kakkilaya, 2004;)
This measure of parasitaemia is used in the study of fever threshold and estimation of fever risk, assessing the efficacy of the therapeutic interventions and in stratifying resistance to antimalaria treatment (Trape et. al., 1985; Ejezie and Ezedinachi, 1992; Genton et. al., 1994; Rogier et. al., 1996; Delley et. al., 2000; Montari et. al., 2001; O’meara et. al., 2005; Cheesbrough, 2006;)
Calculating parasite density is also important for estimating herd immunity to malaria and for determining end point for interventions such as malaria vaccines, impregnated bed nets and chemosuppression (Schellenberg et. al., 1994; Petersen et. al., 1996; Montanari et. al., 2001; O’meara et. al., 2005)
Visual inspection of blood smears by light microscopy is the method available for quantifying malaria parasite density, doing so by comparing the ratio of counted parasites within a given number of microscopic fields (hpf) against either counted white blood cells (WBC) or counted red blood cells (RBC) within these same fields and then multiplying the ratio by either the measured or estimated density of WBCs or RBCs in the patients’ blood (O’meara et. al., 2005)
It is possible that total parasite quantity present, largely determines pathological and clinical manifestation of malaria, yet peripheral measurement of parasites density show a subset and not total parasite load (Armstrong-Schelenberg et. al., 1994; Delley et. al., 2000).
Above observations suggest that consistency of technique and time of blood collections around noon (12.00-13.00 hours) should give a fairly high and reliable estimation of malaria parasite burden (David et. al., 1983; Grovenor et. al., 1998;).
Relating malaria parasite density to fever onset appears to be a major clinical implication of studies on malaria parasite density. Malaria parasite density thresholds have been proposed for the development of fever due to malaria and it appeared to vary from study to study (Miller,1985; Troupe et. al., 1985; Velema et. al., 1991; Rogier et. al. ,1996).
It is important to note that very high parasite levels >100000/uL can exist in persons with no symptoms at all, this is apparently a result of premonition (Hogh, et. al., 1983; Hogh, 1996). Parasite density needed to trigger fever strongly varies from person to person, reflecting the possible role of immune factors on fever threshold (Rogier et. al. 1996; Delley et. al., 2000)
An age dependent threshold effect of parasite density on occurrence of fever was described; the threshold varied from a maximum of 2.45 trophozoites /leucocytes at 1 year of age and a minimum of 0.5 trophozoites /leucocytes at 60 years of age. When an individual parasite density crossed the threshold level corresponding to his/her age , the risk of fever multiplied by 44 (Rogier et. al., 1996).
Irrespective of appearance or not of symptoms,asymptomatic carriers of malaria parasites constitute a significant reservoir indispensable in any program to eradicate this pernicious disease. (Aguas et al, 2008)
The vector of malaria is the female Anopheles mosquito and factors favoring them their breeding and contact with humans include garbage heaps ,swamps and stagnant pools of water that are close to houses as well as type of house (cement bulk are more protective than wood-plank houses) and state of mosquito nets (Nkuo-Akenji et. al., 2006). According to MARA (Mapping Malaria Risk in Africa ) sponsored project which based its findings on the Garki project(Molinueax and Gramicia, 1980) the only environmental co-variant from the water significantly related to transmission intensity was distance from the water indicating high transmission in areas within 4 kilometers of water source (Gemperli et. al., 2006).
Control measures in families in Nigeria have been studied. Children in families who used insecticides with coils as preventive measure against malaria ,has the highest infection rate 50% followed by those using insecticides alone (37.2%) , nets (35.1%), insecticides with nets (33.8%) coils alone 31.6%. those who used none of the above measures had a rate of 31.6%. Those who used none of the above measures had a rate of 30.7% and those who used all had a rate of 0% (Adeoye et. al., 2001). The importance of environmental factors can again be highlighted by the Zambian experience, where multiple control interventions including environmental management against anopheles larval stages and improvement in hygiene and sanitation reduced the overall malaria incidence and mortality rates by about 50% (Utzinger et. al., 2001). However I did not find any studies on control measures and entomological inoculation among students in Delta state of Nigeria.
The public health impact of malaria control interventions such as the use of mosquito net/insecticide treated net is estimated drawing from areas of stable malaria transmission(Lengeler C, 2004; Phillips-Howard et al, 2003). Such estimations are used to assess the impact of use of nets on disease burden.
In an area of stable malaria transmission, residents often observe mosquito bite nodules in the mornings at waking up. In such an area like Abraka, southern Nigeria, residents have taken to installation of mosquito nets on all shutters including the doors and this is usual practice in several privately owned hostels in the university village. In this study perception of mosquito bite nodules on waking was used as a measure of entomological inoculation and this was expected to be inversely proportional to level of use of the control nets and parasite density.
Frequency of use of anti-malaria medication is an indication of how often an individual develops symptoms suspected to be malaria. The usual perspective in areas of stable transmission in the absence of laboratory confirmation is that any fever is considered to be malaria unless there is another obvious cause, so individuals tend to purchase and take anti-malaria medication on their own (Diallo et. al., 2006).
Abraka is a semi-urban town located in the Niger-Delta region of Nigeria between latitude 5 degrees, 30 minutes and latitude 6 degrees north of the equator and longitude 6 degrees and 6 degrees, 30 minutes from the Greenwich meridian. The height above sea level is between 50-200 metres. Abraka is covered by rainforest vegetation and receives not less than 4000mm of rain annually with the rainy season starting as early as late January-early February and dry season between late November and early December. The indigenes are Urhobo by tribe and it is host to the main campus of the multi campus state university; Delta State University. The fresh water River Ethiope runs through the town very close to the university hostels. Being a university town and yet semi urban, it is host also to students from different parts of the country who live predominantly in privately owned hostels most of which fall grossly below expected housing standards. Several beach resorts line the course of the river with attendant diversion of water to create pools for recreation. Poor housing and proximity to stagnant water body are known factors in the transmission and prevalence of malaria (Nkuo-Akenji et. al., 2006). This study was carried out in July-August 2008.
Aim and Objectives
This study aims to provide baseline data of the indices of malaria prevalence in the study population as well as other malariometric indices namely use of mosquito nets, subjective observation of mosquito bite nodules, parasite density.
The objectives include an assessment of frequency of use of mosquito-net among students of Delta State University, Abraka using the study sample. Frequency of observation of mosquito bite nodules among the volunteers, frequency of useof anti-malarial medication in the last three months and malaria parasite density associated with level of use of mosquito nets.
This study will help assess the impact of efforts to roll back malaria in the study population.
Materials and Methods
Sampling
A random sample of students of College of Health Sciences Delta State University, Abraka and students presenting for health check and treatment at the university health centre were studied. Sample size calculation using an estimated prevalence rate among students at Eku, Delta State (Nmor and Egwunyenga, 2005) of 11.76% and with a maximum sampling error of 5%. Estimated minimum sample size called for was 159 students. However due to difficulty in obtaining informed consent from asymptomatic students willing to have their blood taken I was just able to obtain samples from 103 students comprising 60 females and 43 males. 93 were asymptomatic (apparently healthy) and 10 were symptomatic. 66 of the subjects lived in off-campus accommodation and 37 within the campuses but all lived within four kilometres of the River Ethiope.
Criteria for Inclusion
Volunteers with no history of fever in the previous two weeks, not on any anti-malaria treatment and not on any antibiotic nor having any objection to being a part of this research having being informed of the purpose, procedure, risks and benefits (informed consent) were recruited for the study. Only asymptomatic subjects were used for this aspect of the study.
Ethical Permission
Ethical permission was sought and obtained from the Director of Health Services, Delta State University, Abraka.
Procedure
A questionnaire was administered at first contact, the first page of which is for informed consent.
Test period for subjects was at contact(week 0), after informed consent. Using a 20 microlitre capillary tubes, capillary blood was obtained from a finger prick between 12.00 noon and 13.00 hours on week 0. A drop of blood was used to make a thin film and three drops of blood was used to make a thick film of diameter about 2.0cm. A slide card was used to standardize the area to be covered by the thick film. Both the thick and thin film was made on the same slide. The thin blood film was air dried and fixed using absolute methanol and stained with Giemsa stain. The thick film shall was air dried and stained using Giemsa stain.
Stained slides were examined using x100 objective, x10 eyepiece and oil immersion. The thick film was used for counting malaria parasites against 200 white blood cells. The thin film was used to identify the species. I identified only Plasmodium falciparum species. The proportion of samples having malaria parasites were calculated as parasite rate. Parasite rate is really an index, of prevalence and is defined mathematically as the proportion of persons sampled who has a positive malaria parasite result divided by the number of persons who provided blood samples, expressed as a percentage ( Delley et. al., 2000; Schiff, 2002; Eisele et. al., 2006; Hay et. al., 2008). Using an estimated white blood cell count of 7000/µl, I calculated parasite density.
Data Analysis
Data was manually analysed and Microsoft excel was used to create the illustrative diagrams.
Results
Female subjects used mosquito nets more often than males (19.3% :16.7%) and male subjects had more frequent mosquito bites than males (72.2%:56.1%). The level of coverage of mosquito net use is 18.3% . 81.7% of the subjects had no mosquito net coverage and out of the, 56.1% see mosquito bite nodules at least each month. 62.4% of the subjects had frequent (> 1/month) mosquito bites and of these 44.8% were male and 55.2% were female, this proportion was not significantly different from a 50:50 distribution. (χ2 at p<0.05) Interestingly no subject reported negative observation of mosquito bite nodules in the past 2 years. Several subjects who had mosquito net coverage still had frequent bite nodules , especially from > 1/month.
Table 1: Age Distribution of 103 Subjects investigated.
| Age Group (years) | Male | Female | Overall | |||
| No | % | No | % | No | % | |
| 15 – 19 | 17 | 16.51 | 13 | 12.62 | 30 | 29.13 |
| 20 – 24 | 21 | 20.39 | 35 | 33.98 | 56 | 54.37 |
| 25 – 29 | 3 | 2.91 | 9 | 8.74 | 12 | 11.65 |
| 30 – 34 | 2 | 1.94 | 2 | 1.94 | 4 | 3.88 |
| >35 | – | – | 1 | 0.97 | 1 | 0.97 |
| Total | 43 | 41.75 | 60 | 58.25 | 103 | 100 |
Table 2: Distribution of average frequency of observation of mosquito bite nodules and use of mosquito nets among male and female asymptomatic subjects.
| Average
Frequency of Mosquito Bite |
Male | Female | ||||
| No | Net Use | No | Net Use | |||
| Yes | No | Yes | No | |||
| <1/year | 6 | – | 6 | 12 | 7 | 5 |
| 1/year | 3 | – | 3 | 6 | – | 6 |
| >1/year | 1 | – | 1 | 7 | – | 7 |
| 1/month | 4 | – | 4 | 6 | 1 | 5 |
| >1/month | 5 | 1 | 4 | 5 | – | 5 |
| 1/week | 7 | 2 | 5 | 12 | 2 | 10 |
| >1/week | 6 | 3 | 3 | 6 | 1 | 5 |
| Daily | 3 | – | 3 | |||
| Total | 36 | 6 | 30 | 57 | 11 | 46 |
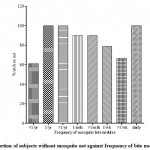 |
Figure 1: Proportion of subjects without mosquito net against frequency of bite nodules.
|
Table 3: Distribution of ABO blood group, genotype and use of anti-malaria drugs (within three months prior to study) among male and female asymptomatic subjects.
| ABO
Blood Group |
Male | Female | Total | |||||
| AA | AS | A/Muse | AA | AS | A/M use | No | %A/M use | |
| A+ | 4 | – | 4 | 19 | – | 16 | 23 | 87.0 |
| B+ | 7 | 2 | 7 | 4 | 1 | 4 | 14 | 78.6 |
| AB+ | – | – | – | 4 | – | 3 | 4 | 75.0 |
| O+ | 24 | – | 18 | 22 | 4 | 14 | 50 | 66.7 |
| A- | – | – | – | – | – | – | – | – |
| B- | – | – | – | – | – | – | – | – |
| AB- | – | – | – | 2 | – | – | 2 | 0.0 |
| O- | – | – | – | – | – | – | – | – |
| Total | 35 | 2 | 29 | 51 | 5 | 37 | 93 | 79.93 |
Parasite rate (slide positivity rate) among asymptomatic subjects at week 0 is 92.5%. Genotype AS harbored higher parasite densities than genotype AA , at density >1000/mm3 , they however used anti-malaria drugs less frequently than genotype AA.
Table 4: Distribution of genotype and parasite density at week 0 among asymptomatic subjects.
| Parasite Density Range (/mm3) | Male | Female | Total | |||
| AA | AS | AA | AS | No | % | |
| 0 – 1 | 1 | – | 6 | – | 7 | 7.5 |
| 1 – 100 | – | – | 2 | – | 2 | 2.2 |
| 101 – 1000 | 16 | – | 11 | – | 27 | 29.0 |
| 1001 – 10000 | 17 | 2 | 31 | 4 | 54 | 58.1 |
| >10000 | – | – | 1 | 2 | 3 | 3.2 |
| Total | 34 | 2 | 51 | 6 | 93 | 100 |
Discussion
The preponderance of young adults aged 15-24 years in this study focuses our research on individuals who are at the peak of their homeostatic reserve and whose immune system are yet to begin to undergo homeostenosis (Resnick, 2001).
Volunteer perception of frequency of observation of mosquito bite nodules is a subjective indication of how often an individual is exposed to mosquito bites. Arguably this is expected to increase with absence of mosquito nets. It however appears that increase in the number of subjects having mosquito netted shutters did not significantly reduce or the frequency of mosquito bites . Oral discussion with several subjects revealed that most noticed the presence of nocturnal insects (usually found in the rainforest regions) inside their rooms despite the netted shutters. On inspection of some such residences, it was noticed that there several gaping spaces between the “mosquito net” panel and the windows. With the relative high atmospheric temperature (mostly 30ocelcius and above) and the very, very poor power supply, many students in a bid to combat the heat, resort to leaving their windows ajar while keeping the ant-burglary and “mosquito netted” panels in place. This is coupled with the fact that malaria parasitaemia increases attractiveness of humans to mosquitoes (Lacroix et. al., 2005) and this is dependent on human sweat components; hot humid environments. Poor infrastructure and climate in this case are drivers for transmission especially where most subjects (92.5%) harbor malaria parasites. This reflects ineffectual mosquito net protection and can account for this observed inconsistency between use of mosquito nets and frequency of mosquito bites. It could also be due to a discrepancy of reporting.
The low coverage of nets indicates the level of this aspect of malaria control activities in a University town in 10 years of the roll back malaria initiative. The Nigeria malaria control program delivered about 17 million insecticide treated mosquito nets during 2005 – 2007 and this was estimated to cover only 23% of the population at risk (WHO, 2008). Moreover even the supplied nets were hardly distributed as they lay stored at local government stores. In an endemic rainforest region, net coverage should be high and effective if there is to be any hope of interrupting transmission and control the intensity of malaria in the Niger Delta. Of note here is the widespread scarcity of insecticide treated mosquito nets for beds; in-fact there are no known pharmacies or markets where this can be purchased in the state at this time.
However, given the protective premunition maintained by the presence of parasites in the blood and the penchant of Nigerian government policies to swing from end to end, the goal to control malaria should be such that still maintains some parasite in the population so that premunition is not lost since that would make previously semi- immune persons to become non-immune. This is because the attendant severe morbidity and mortality associated with falciparum malaria in non-immune persons is best avoided.
Whereas 70.97% of subjects had used anti-malaria drugs within the last three months, 81.7% had no mosquito net coverage. This leaves a small percentage-10.73% who had no mosquito coverage yet had no malaria attacks in the last three months. This 10.73% represents the effects of anti-malaria immunity.
The infrequency of use of anti-malaria drugs by subjects who are HBAS heterozygote (sickle cell trait) concurs with what is known, that HBAS carriers have a sixfold reduction in the risk of severe falciparum malaria (White and Breman, 2001).
The parasite rate (slide positivity rate) at the first contact with respondents (week 0) is 92.5%. This is agrees with the study in northern Nigeria (Engelbrecht et. al., 2000) which found a prevalence rate of 94.2%, and comparable to the studies in pregnant women in southwestern Nigeria like Osogbo—88.2% (Adefioye et. al., 2007) and Ibadan—73% (Onyenekwe et. al., 2005). It is however higher than the value obtained at the nearby town of Eku over an eight month period (Nmor and Egwunyenga, 2005) which found an overall prevalence rate of 69.4% and a rate of 11.76% for students. Prevalence of malaria parasitaemia have been shown to fluctuate with season and to be highest at the height of the rainy season at which time the present study was carried out (Rogers and Randolf, 2000; Delley et. al., 2000; Feachem and Sabot, 2007; Hay et. al., 2008). The parasite density at week 0 ranged from 84/mm3 in a female respondent who was protected by mosquito nets to 63142/mm3 in a female respondent who was not protected by mosquito nets.
Conclusion
There is low mosquito net coverage in this university village in southern Nigeria ten years into roll back malaria. This is associated with high and frequent use of anti-malaria medication and frequent observation of mosquito bite nodules. Even where there are mosquito netted shutters, coverage was noted to be often ineffectual leading to high reports of entomological inoculation. This study documents impacts of efforts to control malaria in an area of stable transmission in southern Nigeria.
References
- Adefioye O.A, Adeyebi O.A, Hassan W.O, Oyeniran O.A.,(2007): Prevalence of malaria Parasite infection among pregnant women in Osogbo southwest Am.-Eur. J. Sci. Res.2(1): 43 – 45.
- Adeoye G.O, Osayemi C.O, Oteniya O, Onyemekeihia S.O., (2007): Epidemiological studies of intestinal Helminths and malaria among children in Lagos, Nigeria . J. Biol. Sci. 10(13): 2208 – 2212.
- Aguas, R., White, L. J., Snow, R. W. and Gomes, M. G. (2008): Prospects for malaria eradication in sub-sahara Africa. Plos One 3(3): doi:1371/journal.pone.0001767.PMID 185555042
- Armstrong-Schellenberg, J. R. M., Smith, T., Alonso, P. L. and Hayes, R. J. (1994): What is clinical malaria? Finding case definitions for field research in highly endemic areas. Today. 10: 439 – 442.
- Cheesbrough M, 2006, District Laboratory Practice In Tropical Countries Part 1, Cambridge University Press, U.K pp: 239-258
- David, P. H., Hommel, M., Miller, L. H., Udeinya, I. J. and Oligino, L. D. (1983) Parasite sequestration in Plasmodium falciparum malaria :spleen and antibody modulation of cytoadherence of infected erythrocytes. Natl. Acad. Sci. USA.80: 5075 – 5079.
- Delley, V., Bouvier, P. and Breslow, N. (2000): What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Med. Int. Health5(6): 404 – 412.
- Diallo, D. and Graz, B. (2006): Malaria treatment in remote areas of Mali. Use of modern and Traditional Medicines; patient outcome. R. Soc. Trop. Med. Hyg. 100: 515 – 520.
- Eisele, T. P., Keating, J., Bonnett, A., London, B., Johnson, D., Lafontant, C. and Krogstad (2006): Prevalence of Plasmodium falciparum infection in rainy season artibonite valley Haiti , Emerging infectious disease .cdc.org/eid.vol 13, no13 Oct 2007 pp1494-1496.
- Ejezie, G. C. and Ezedinachi, E. N. (1992): Malaria parasite density and body temperature in children under 10 years of age in calabar, Nigeria; Geog. Med., 44(1-2) : 97-101.
- Engelbrecht, F., Togel, E., Beck, H. P., Enwezor, Oettli, A. and Felger, I. (2000): Analysis of Plasmodium falciparum infections in a village in northern Nigeria : determination of msp2 genotypes and parasite-specific IgG responses; Trop.jan 5;74(1) : 63-71.
- Feachem, R. G. and Sabot, O. J. (2007): Global malaria control in the 21st century : A historic but fleeting opportunity JAMA297: 2281
- Gemperli, A., Vounatsou, P., Sogoba, N. and Smith D.L.,2006): Malaria mapping using transmission models: Application of survey data from Mali. J. Epidemiol. 163 (3): 289 – 297.
- Genton, B., Smith, T. and Baea, K. (1994): Malaria: how useful are clinical criteria for improving the diagnosis in a highly endemic area? R. Soc. Trop. Med. Hyg. 88: 537 – 541.
- Gravenor, M. B., Hensbroek, M. B. V. and Kwiatkowski, D. (1998): Estimating sequestered parasite population dynamics in cerebral malaria. Natl. Acad. Sci. USA. 95(13): 7620 – 7624.
- Hay, S. I., Smith, D. L,. and Snow, R.W. (2008): Measuring malaria endemicity from intense to interrupted transmission. Lancet infect. Dis8: 369 – 378.
- Hogh, B. (1996): Clinical and Parasitological studies on immunity to Plasmodium falciparum malaria in children; J. Infect. Dis. Suppl; 102: 1 – 53.
- Hogh, B., Marbiah, N. T., Peterson, E., Dolopaye, E., Wilcox, M., Bjokjman A., (1993): Classification of clinical falciparum malaria and its use for the evaluation of chemosuppression in children under six years of age in Liberia, West Africa. Trop. 54: 105 – 115.
- Kakkilaya, B. S. (2004): Evaluation of cases of malaria; The malaria site-http://www.malariasite.com/malaria/Evaluation.htm
- Lacroix, , Mukabana, W. R., Gouagna, L. C. and Koella, J. C. (2005): Malaria infection increases attractiveness of humans to mosquitoes; PLoS Biol3(9):e298doi:10:1371/journal.Pbio.0030298
- Lengeler C.(2004): insecticide-treated bed nets and curtains for preventing malaria. The Cochrane database of systematic reviews 2003, issue 2. Art. No.:CD000363.pub2.DOI:10.1002/14651858.CD000363.pub2
- Miller, J. (1958): Observation on the natural history of malaria in the semi-resistant west Africa. R.Soc. Trop. Med. Hyg. 52: 152 – 168.
- Molineuax, L. and Gramicia, G. (1980): The Garki Project. Research on the epidermiology and control of malaria in the Sudan Savannah of west Africa. World Health Organization, Geneva. 198; pp311
- Montanari, R. M., Bangali, A. M., Talukder, K. R., Baqui, A., Mahswary, N. P., Gosh, A., Rahman, M. and Mahmoud, A. H. (2001): Three case definitions of malaria and their effect on diagnosis, treatment and surveillance in Cox’s bazaar district, Bangladesh. Bull World Health Organisation. 79: 648 – 656.
- Nkuo-Akenji, T., Ntonifor, N. N. and Ndukum, M. B. (2006): Environmental factors affecting malaria parasite prevalence in rural Bolifamba, South-West Cameroun; J. Health Sci. 13(1-2): 40 – 46.
- Nmor, J. C. and Egwunyenga, A. O. (2005): Malaria and trypanosomiasis among blood donors of Delta State, Southern Nigeria. J. Sci. Res. 11(3): 364 – 370.
- O’Meara, W. P., Mckenzie, F. E., Magill, A. J., Forney, J. R., Permpanich, B., Lucas, C., Gasser, Jr R. A. and Wongsrichanalai, C. (2005): Sources of variability in determining malaria parasite density by microscopy. Am J. Trop. Med. Hyg. 73(3): 593-598.
- Onyenekwe, C. C., Meludu, S. C., Arinola, O. G. and Salimonu, L. S. (2005): Relationship between Plasmodiumfalciparum density, haptoglobulin, transferring and packed cell volume in apparently healthy pregnant women. J. Biomed. Res. 18(1): 21 – 24.
- Peterson, E., Marbiah, N. T., New, L. and Gottschau, A. (1996): Comparison ot two methods for enumerating malaria parasites in thick blood films. J. Trop. Med. Hyg. 55(5): 485 – 489.
- Phillips-Howard PA, Nahlen BL, Kolczak MS, Hightower AW, Ter Kuile FO, et al. (2003) Efficacy of permethrin-treated bed nets in prevention of mortality in young children in annarea of high perennial malaria transmission in western Kenya. Am J Trop Med Hyg 68:23-29
- Resnick, N. M. (2001): Geriatric medicin,eIn: Braunwald E, Hauser S.L, Fauci A.S, et. al. (eds) Harrison’s Principles of Internal Medicine, 15th ed, McGraw hill, U.S.A, pp 37
- Rogers, D. J. and Randolf, S. E. (2000): The global spread of malaria in a future warmer world. Sci. 289: 1763 – 1766.
- Rogier, C., Commenges, D. and Trape, J. F. (1996): Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitaemia in highly endemic populations; J. Trop. Med. Hyg. 54(6): 613 – 619.
- Rogier, C., Commenges, D. and Trape, J. F. (1996): Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitaemia in highly endemic populations; J. Trop. Med. Hyg. 54(6): 613 – 619.
- Schellenberg, J., Smith, T., Alonso, P. L. and Hayes, R. J. (1994): What is clinical malaria : finding case definitions for field research in highly endemic areas . Today10: 349 – 442.
- Schiff, C. (2002): Integrated approach to malaria control. Microbiol. Rev ;15: 278 – 293.
- Trape, J. F., Peelman, P. and Morault-Peelman, B. (1985): Criteria for diagnosing clinical malaria among a semi-immune population exposed for intense and perennial Trans. R. Soc. Trop. Med. Hyg. 79: 435-442.
- Utzinger, J, Tozan A. and Singer B. H. (2001): Efficacy and cost effectiveness of environmental management for malaria control. Med. Int. Health. 6: 677 – 687.
- Velema, J. P., Alihonou, E. and Chippaux, J. P. (1999): Malaria morbidity and mortality in children under three years of age on the coast of Benin, west Africa. R. Soc. Trop. Med. Hyg. 85: 430 – 435.
- White, N. J. and Breman, J. G. (2001): “Malaria and babesiosis: diseases caused by red blood cell parasites” In: Braunwald E, Hauser S. L, Fauci A. S, Longo D.L, Kasper D.L, Jameson J.L, (eds). Harrison’s Principles of Internal Medicine 15th ed, McGraw Hill,U.S.A , pp 1203-1209

This work is licensed under a Creative Commons Attribution 4.0 International License.





