Manuscript accepted on : 15 March 2012
Published online on: --
Lilian Ebele Chris-ozoko (Nee Ebite)
Department of Anatomy, Delta State University Abraka, Nigeria.
ABSTRACT: Zidovudine is a type of medicine called a nucleoside reverse transcriptase inhibitor (NRTI). It works by disrupting one of the early steps in the HIV life cycle, called reverse transcription. Twenty (20) adult wistar male rats, with an average weight of 150grams were used for this study. They were divided into four (4) groups made up of five (5) rats each,. Daily consumption of 0.3mg/ml,1.3mg/ml,and 2.3mg/ml of zidovudine, in consonance with the administration by Sikka et al (1991), was administered orally to rats in groups two (2) (subnormal dose group), three (3) (normal dose group), and four (4) (excess dose group) respectively for thirty days using an Orogastric cannula, while rats in group one were administered distilled water only. The rats were also weighed weekly, for four weeks.
KEYWORDS: Zodpvidome; Wistar Rats
Download this article as:| Copy the following to cite this article: Chris-ozoko L. E (Nee Ebite). Effect of Dose Dependent Administration of Zidovudine on the Weight of Wistar Rats (Rattus novergicus). Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Chris-ozoko L. E (Nee Ebite). Effect of Dose Dependent Administration of Zidovudine on the Weight of Wistar Rats (Rattus novergicus). Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9607 |
Introduction
Zidovudine is a type of medicine called a nucleoside reverse transcriptase inhibitor (NRTI). It works by disrupting one of the early steps in the HIV life cycle, called reverse transcription.(Aids infodrug database 2011), its uses include treatment of HIV infection and prevention of maternal-foetal transmission of HIV. Human immunodeficiency virus (HIV) is a lentivirus (a member of the retrovirus family) that causes acquired immunodeficiency syndrome (AIDS), Douek et al.,(2009), a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Infection with HIV occurs by the transfer of blood, semen, vaginal fluid, pre-ejaculate, or breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells. Chronic, high-dose therapy with AZT is associated with significant side effects, including anemia, neutropenia, hepatotoxicity, cardiomyopathy, and myopathy. Minor side effects include nausea and vomiting, headache, changes in the distribution of body fat, sleep disruption and loss of appetite .
The aim of the study is to find the effect of a dose dependent administration of zidovudine on the weight of wistar rats.
Materials and Method
Twenty (20) adult wistar male rats, with an average weight of 150grams were used for this study. They were divided into four (4) groups made up of five (5) rats each, fed growers mash, given water liberally and acclimatized for a week. Daily consumption of 0.3mg/ml,1.3mg/ml,and 2.3mg/ml of zidovudine, in consonance with the administration by Sikka et al (1991), was administered orally to rats in groups two (2) (subnormal dose group), three (3) (normal dose group), and four (4) (excess dose group) respectively for thirty days using an Orogastric cannula, while rats in group one were administered distilled water only. The rats were also weighed weekly, for four weeks.
Data analysis used includes mean and standard deviation.
Results
Mean Weight Of Animals (Expressed In Mean ±Sd)
| GROUPS | INITIAL | WEEK 1 | WEEK 2 | WEEK 3 | WEEK 4 |
| CONTROL | 151.00±8.22 | 152.60±7.92 | 154.60±6.58 | 158.00±6.28 | 159.00±8.12 |
| SUBNORMAL | 153.33±9.83 | 158.40±14.12 | 158.60±14.19 | 157.20±15.06 | 156.60±15.01 |
| NORMAL | 152.50±6.12 | 151.00±5.66 | 152.40±5.13 | 152.00±5.70 | 151.60±5.68 |
| EXCESS | 162.00±19.56 | 169.80±21.51 | 161.80±21.32 | 162.40±20.28 | 161.20±19.69 |
Control showed steady increase in weight through wk 1,2 and 3., the subnormal dose, had an initial significant rise in in the first week which was maintained at week 2 then a slow drop. The rats with normal dose first had a significant drop in weight then a steady increase after from week 2 to 3 to normal levels then a drop at the fourth week., the rats with excess dose showed a rapid drop below normal then a slight increase again at week 3 then a slow drop at week 4.
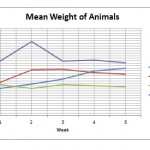 |
Figure 1
|
Discussion
The results above at best shows the the effect of zidovudine on the weight of wistar rats is erractic. There was clear evidence of a dose dependent effect on the weight of the adult wistar rats. . diifferents studies have expressed different opinion of the effect of zidovudine on weight, some author claim it increases weight gain by decreasing the number of opportunistic infections and improving T4(CD4) lymphocyte count (the Columbia electronic encyclopeadia 2007). In our study all the rats except those at normal dose of the drug showed an initial increase in weight, . That is weight gain observed occurred only in those at extremes of dosage (subnormal and excess dosages) . This initial increase could also be physiological due to the growers marsh they were fed, since this were uninfected rats, Hence the continous growth noticed in the control group. Those with subnormal dose of the drugs started loosing weight at week 3, the effect on those at normal dose was more interesting at the first week there was a drop in weight. After which there was an increase.at the 3rd week then a drop thereafter, this could be manisfestation of toxicity after the initial adaptation
[sahaj et al 1994] in Canada found no significant difference in body weight and clearance rate of zidovudine. In HIV infected and non infected men. we also observed that the average weight of the group with normal doses was lower than those of the other groups treated with the drug probably showing a dose dependent response based on early toxicity probably because of the lower surface available for absorption area in this groups of wistar rats. This agrees with the study by willig et al 2010 who observed an increased risk of early toxicity related discontinuation of AZT containing containing regimen for baseline weight of individuals less than <60kg and also an early toxicity related discontinuation of AZT in patients in 44 % of patients < 50kg and only 14% in patients >70kg. Though there is need for further studies on both infected and non infected mammals to further validate this fact. We however also noticed the appearance of sores on the mouths and tails of all rats in the excess group on the fourth day of the second week of AZT administration. There was a reduction in water and food intake for all the groups on AZT. Further emphasizing the role toxicity probably has on the manisfestation of side effects.From this study we can conclude that the effect of zidovudine on wistar rats is both weight dependent and duration of administration dependent ; both, a probable function of toxicity. We would also advised that weight dependent administration of the drug as is done for children be carefully formulated, administered and observed in adults too to encourage compliance and increase regimen durability.
References
- Douek C., Roederer M., and Koup R.A. (2009). “Emerging concepts in theimmunopathogenesis of AIDS”. Annu. Rev. Med. 60.pp. 471–84.
- Sahai J, Gallicano K, ormsby E, Garber G, Cameron DW.(1994) Relationship between body weight , body surface area and serum Zidovudine pharmacokinetic parameter in adult , male HIV- infected patients. Aids, jun;8(6):793-6
- Suresh C. Sikka,Sudhir R. Gogu and Krishna C. Agrawal (1991).Effect of zidovudine (AZT) on reproductive and haematopoietic systems in the male rat.Biochemical pharmacology 42(6): Pp 1293-1297
- The Columbia electronic encyclopeadia 6th Copyright 2007 columbia university press. Available at http://www.encyclopedia.com/topic/AZT.aspx accessed 28th of February 2011.
- Wikipedia contributors. Zidovudine. Wikipedia, The Free Encyclopedia. February 29, 2012, 04:04 UTC. Available at: http://en.wikipedia.org/w/index.php?title=Zidovudine&oldid=479415557. Accessed March 2, 2012.
- Willig JH,Echevarria J, Westfall AO, Iglesias D,,Henostroza G, Seas C, Mugavo MJ, et al., (2010) durability of initial antiretroviral therapy in a resource –consrained setting and the potential need for zidovudine weight –based dosing. J.acquired immune defic syndr.53(2):215-21

This work is licensed under a Creative Commons Attribution 4.0 International License.





