Manuscript accepted on : 28 February 2012
Published online on: --
Bioactivities of Protein Isolated from Marine Sponge Zygomycaleparishii
K. V. Anitha
Malankara Catholic College, Mariagiri, Kaliakkavilai, K.K. Dist -629 153, India.
Corresponding Author E-mail: manjusha_aj@yahoomail.co.in
ABSTRACT: Today, one emerging source of small molecule drug lead in the world's ocean. Based on the present findings, the marine sponge extract from Zygomycaleparishi have potential antimicrobial activity against pathogenic bacteria and fungi. Methanolic extract of sponge Zygomycaleparishiishowed three bands ranging from 70-150 kDa and aqueous crude extract of Zygomycaleparishiirevealed three bands which ranging between 66-205 kDa on SDS-PAGE.The results of present investigation revealed that,the marine sponges are a potential source of novel antibiotic leads.
KEYWORDS: Marine sponge; Zygomycaleparishi; Antimicrobial activity; SDS-PAGE
Download this article as:| Copy the following to cite this article: Anitha K. V. Bioactivities of Protein Isolated from Marine Sponge Zygomycaleparishii. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Anitha K. V. Bioactivities of Protein Isolated from Marine Sponge Zygomycaleparishii. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9732 |
Introduction
The ocean is said to be the medicine chest of the future and the marine sponges plays vital role among them. The marine environment is an exceptional reservoir of bioactive natural products, which produce several novel structures with unique biological properties. Among the groups of marine organisms sponges are the most diverse and abundant, due to their soft bodies and sedentary life styles. The presence of large amounts of microorganisms within the mesohyl of many demosponges has been well documented (Hentschelet al. 2002; ImhoV and Stöhr2003). Sponge mesohyl was referred as “micro-environments” providing a broad variety of ecological niches (Thiel et al. 2007). Bacteria can contribute up to 40% of the sponge biomass (equal to about 108 to 109 bacteria g tissue¡1) and are probably permanently associated with the host sponge unless they are disturbed by external stress factors (Friedrich et al. 2001; Thomset al.2003; Webster and Hill 2001).Presently, occurrence or new infectious diseases and development of drug resistant pathogens are threatening the living organisms. More number of antimicrobial substances developed so far was impractical to tackle the situation, because of their complex chemical nature, (that produceenvironmental consequences) and the development of resistant pathogenic strains. So the present study is in urge, to develop ecofriendly, easily available, cheap antimicrobial compound.
Materials and methods
Sponge was collected by scuba divers from South west coast of India. Voucher specimens were either preserved in 70% ethanol immediately upon collection or freeze-dried for chemical characterization. Freeze-dried specimens were prepared for histology by dehydration in a very weak solution of detergent for 24 h, followed by preservation in 70% ethanol. Histological sections and spicule preparations were made as in the work of Kelly-Borges and Vacelet (1995).
The sponges collected in methanol containers were squeezed in a tissue homogenizer, depending on the nature of the sponge species, which was used for extraction. In the case of Mycale species , they were cut into small pieces, and squeezed to prepare the crude extract using a mortar and pestle, using methanol as solvent . They were extracted thrice and the combined extract was concentrated in a rotary vacuum evaporator rotary vacuum evaporator (Buchi, Flawil, Switzerland) at room temperature. Thus concentrated crude extract was collected in air tight plastic containers and kept in refrigerator.The aqueous extract of sponge was prepared by squeezing the sand- free specimens in triple distilled water. The resultant solution was filtered and dialyzed, using dialysis membrane against D-glucose to remove the excess water. The supernatant obtained was lyophilized and stored at 40C in a refrigerator for further use as aqueous crude extract for SDS-PAGE.
The antimicrobial susceptibility testing was done by Kirby-Baeur disc diffusion method. This method allows the rapid determination of the efficacy of a drug by measuring the diameter of the zone of inhibition that result from diffusion of the agent into the medium surrounding the disc. In this procedure, filter paper discs of uniform size (6mm) are impregnated with specified concentrations of two methanolic sponge extract and then placed on the surface of an agar plate that has been seeded with the organisms to be tested. The medium of choice is Mueller-Hinton agar, with pH of 7.2 to 7.4, which is poured into plates to uniform depth of 5mm and refrigerated on solidification. Label the covers of each plates with the name of test organisms to be inoculated ie.,Acetobactersp(MTCC2903), Bacillus cereus. (MTCC430), Bacillus subtilis (MTCC441) ,Gluconobacteroxydans (MTCC904), Pseudomonas aeruginosa (MTCC741) obtained from Microbial Type Culture Collections (MTCC), Chandigarh, India. Bacterial colonies were allowed to grow overnight at 370C , then the inhibition zone around the disc was measured (Cappucinoet al., 2004).
Petri dishes with Rose Bengal Agar medium were inoculated Aspergillusaculeatus(MTCC1331),Aspergillusniger(MTCC281), Candida tropicalis(MTCC1000),.Rhizomucormicchii (MTCC546) obtained from Microbial Type Culture Collections (MTCC), Chandigarh, India. Round paper discs of 6mm were dipped in to 0.001 mL of each methanolic sponge extract and placed in the centre of the inoculated petri dishes. Fungal colonies were allowed to grow overnight at 20ºC and then the inhibition zone around the disc was measured.
The crude extracts were purified by ammonium sulphate precipitation. During ammonium sulphate precipitation, the salt has to be prevent increase of high local concentration. Ammonium sulphate was used for precipitation of total proteins at -90% saturation or for precipitation of proteins using different saturations of salt 40% saturation was done to precipitate the proteins from the culture. The solution was equilibrated for approximately one day in cold condition to ensure complete precipitation and then the precipitate was collected by centrifugation and it was further purified by dialysis method using dialysis membrane. Thus prepared were used for One dimension Sodium Dodecyl Sulphate (SDS) polyacrylamide Gel Electrophoresis (PAGE) was carried out. The protein content of methanolic crude extract and aqueous crude extract were dissolved in 300µl of sample buffer and the samples were loaded into several lanes of 30% gradient gel along with the molecular weight standards. The process adapted a power supply of 80 volts for 3 hours. After electrophoresis, the gel was stained with silver staining (Laemmli, 1970).
Results and Disscussion
Present study revealed that the tested in marine spongeZygomycaleparishipossessed potential antibacterial activity against Acetobactersp , Bacillus cereus, B. subtilis, Glucanobactoroxydens, Pseudomonas aeruginosa (Fig.1). When tested by the disc diffusion method, methanolic sponge extract of Zygomycaleparishishowed significant activity against B. subtilis and Glucanobactoroxydens produced minimum inhibitory activity 19mm. Among the five species of bacteria sponge methanolic extract showing highly significant antibacterial activity against P. aeruginosa .
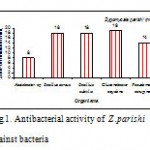 |
Figure 1: Antibacterial activity of Z .parishi against bacteria.
|
In antifungal activity it was found that the metanolic extract successfully prevent the growth of fungi (Fig.2). The inhibitory zone produced by extract against Aspergillusaculeatus 12mm ,Aspergillusnigeris 13mm, Candida tropicalisis 9mm andRhizomucormicchii is 14mm. Among this Zygomycaleparishiihas more activity against Rhizomucormicchii(14mm) followed by Aspergillusniger(13mm) and Candida tropicalis(9mm) . The extract shows feeble activity againstAspergillusaculeatus(12mm).
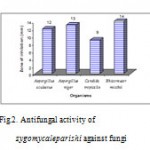 |
Figure 2: Antifungal activity of zygomycaleparishi against fungi.
|
In the present study, sponges ZygomycaleParishicollected from Vizhijam coastal area, produced potent antimicrobial extracellular products. This property indicates that the species might have defense mechanisms in the host sponge. These sponges has extreme potent of antifungal and antibacterial activity (Chelossiet al., 2006). Bergquist and Bedford (1978) have been reported that the antibacterial agents produced by sponges may have a role in enhancing the efficiency with which sponge retain bacterial food and also reported that the activity was higher in temperate species than tropical species (87% as opposed to 58%) and the sponge extract more frequently inhibited the growth of marine bacteria. extracts of different sponge species show activity on various microorganisms, the obtained results suggest that some sponges are seemed to be pathogen specific, which are active against the bacteria (Clathriasp., Sigmodociasp., Callyspongiasp.) and some are against the fungi (Callyspongiasp., Zygomycalesp.)(Rajagopalet al.,2008). Faulkner et al. (2001) have stated that marine sponges have a potential to provide future drugs against important diseases, such as malaria, cancer and a range of viral diseases. Rifaiet al. (2004) isolated Untenospongin B from the marine sponge Hippospongiacommunisand tested for its antimicrobial activity against bacteria and human pathogenic fungi using agar disk method and found to possess a broad and strong activity toward the test organisms.
SDS-PAGE analysis revealed the presence of bands in them. Methanolic extract of spongeZygomycaleparishiishowed three bands ranging from 70-150 kDa. Aqueous crude extract of Zygomycaleparishiirevealed three bands which ranging between 66-205 kDa. It indicates that the fractions might have been responsible for the potent antimicrobial activity(fig.3.).
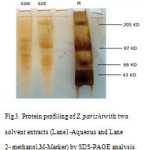 |
Figure 3: Protein profiling of Z. parishiwith two solvent extracts (Lane1-Aqueous and Lane2- methanol,M-Marker) by SDS-PAGE analysis.
|
Selvin and Lipton (2004) and Kanagasabhapathyetal. (2004) have also confirmed that the sponge species of the southern Eastern Peninsular Indian Coast are the ideal candidates for the production of various antimicrobial (bacterial and fungal) and antifouling drugs. In the present study, the SDS-PAGE on the 30% gradient gel, the crude protein toxins yielded 3 bands each in the methanolic extract and aqueous crude extract of Z .parishi. The secondary metabolites metabolites of host sponge D. nigra have demonstrated broad spectrum antibacterial activity and inhibited the growth of all tested bacteria (Selvinet al.,2004a). Culture-based studies indicated that the supplementation of host sponge extract in the culture media drastically increased the number of morphotypes (Selvinet al.,2004b). Sponge symbionts are thought to benefit their hosts in many ways (Wang, 2006;Taylor et al., 2007). This study revealed the presence of potentially new bacterial phylotypes in M. armata(Wang et al.,2008). Based on the findings, it could be inferred that sponges could form a reliable source for bioprospecting of next generation pharmaceutical agents.
References
- Adriana, , ThomazVieiralves, Flavia R.M. Lamarao, Leonardo L.M. Assumpcao, Debor Gomes, LiaJascone Ana LuizaValadao, Rodolpho M. Albano, Gisele Lobo-Hajdu. (2007) Field preservation and optimization of a DNA extraction method for Porifera.Porifera Research: Biodiversity, Innovation and Sustainability. pp: 555-560.
- Bergquist, P.R. and Bedford, J.J (1978) The incidence of antibacterial activity in marine Demospngie, Systemic and Geographic consideration. M ar. Biol. 46: 215-221.
- Cappucino, Natalie Sherman. (2004) The Kirby –Bauer Antibiotic Sensitivity Test. Microbiology, A laboratory manual. Sixth edition. pp:264-266.
- Chelossi, E., Mancini, I., Sepcic, K., Turk, T., Faimali, M. (2006) Comparitive antibacterial activity of polymeric 3-alkylpyridinium salts isolated from the Mediterranean sponge Renierasarai and their synthetic analogues. Eng. 23:317-323.
- Faulkner, A.D., Wright, G., Matthee, F. and Konig, G.M. (2001) Culture extracts of sponges showed antifungal, antialgal and antibacterial activity and cytotoxicity. Mycological Research.
- Friedrich A.B, Hacker J, Fischer I, Proksch P, Hentschel U (2001) Temporal vaiations of the microbial community associated with the Mediterranean sponge Aplysinaaerophoba. FEMS MicrobiolEcol 38:105–113.
- Hentschel U, Hopke J, Horn M, Friedrich AB, Wagner M, Hacker J, Moore BS (2002) Molecular evidence for a uniform microbial community in sponges from diVerent oceans. Appl Environ Microbiol 68:4431–4440.
- ImhoV JF, Stöhr R (2003) Sponge-associated bacteria, general overview and special aspects of bacteria associated with Holichondriapanacea. In: Müller WEG (ed) Molecular marine biology of sponges. Springer, Berlin.
- Kanagasabhapathy, M., Nagata, K., Fujita, Y., Tamura, T., Okamura, H. and Nagata, S.( 2004) Antibacterial activity of the marine sponge Psammaplysillapurpurea: Importance of its surface-associated bacteria. Proceedings of Oceans’04. Techno-Ocean,04: 1323-1329
- Laemmli UK. (1970)”Cleavage of structural proteins during the assembly of the head of bacteriophage T4″. 227 (5259): 680–685.
- Liu, P.V. (1957) Survey of hemolysin production among species of Pseudomonas. J. Bacteriol. 74, 718–727.
- Mitra, A., Santra, S.C. and Mukharjee, J. (2008) Distribution of actinomycetes, their antagonistic behavior and the Physio-chemical characteristic of the worlds largest tidal mangrove forest. Applied Microbial Biotchnol., 80: 685-695.
- Rajagopal, B., S.R. Jegan, R.V. Seena, S. Suma, T.K. Angiesh and Baby Joseph. (2008) Bioactive screening of Peninsular Indian Marine sponges on selected microorganisms. Journal of basic and applied biology. 2 :56-64.
- Rifai, S., Fassouane, A., Kijjoa, A. and Soest, R.V.(2004) Antimicrobial activity of untenospongin B, a metabolite fro the marine sponge Hippospongiacommunis collected form the Atlantic coast of Morocco. Marine Drugs, 2: 147 – 153
- Saadoun, I. and A. Muhana, (2008) Optimal production condition, extraction, partial purification and characterization of inhibitory compound(s) produced by Streptomyces Ds-104 isolate against Multi-drug resistant Candia albicana. Curr. Trends. Biotechnol. Pharm. 2: 402-420.
- Samuel, M., Lu, M., Pachuk, C.J ,Satishchandran, C. (2003).A spectrophotometric method to quantify linear DNA. 313: 301-306.
- Schwartsmann, G., Da Rocha, A.B., Mattei, J., Lopes, R. (2003) Marine- derived anticancer agents in clinical trials. Expert OpinInvestig Drugs. 12:1367-1383.
- Selvin, J. and Lipton, A.P. (2004) Biopotentials of secondary metabolites isolated from marine sponges. Hydrobiologia 513, 231–238.
- Selvin,J , Soniya Joseph, Asha, Manjusha, Sangeetha,Jayaseem, Antony, (2004) Antibacterial potential of antagonistic Streptomyces sp. isolated from marine sponge Dendrillanigra .FEMS Microbiology Ecology, 50(2): 117–122.
- J. and Lipton, A.P. (2004) Antimicrobial activities of marine sponges collected off the coast of Santa Catarina – Biopotentials of secondary metabolites. Hydrobiologia, 513: 231-238.
- Taylor MW, Radax R, Steger D, Wagner M. (2007). Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. MicrobMolBiol Rev 71: 295–347.
- Thiel V, Neulinger SC, Staufenberger T, Schmaljohann R, ImhoV JF (2007) Spatial distribution of sponge-associated bacteria in the Mediterranean sponge Tethyaaurantium. FEMS MicrobiolEcol 59:47–63.
- Thoms C, Horn M, Wagner W, Hentschel U, Proksch P (2003) Monitoring microbial diversity and natural products proWles of the sponge Aplysinacavernicolafollowing trasplantation. Mar Biol 142:685–692.
- Wang G. (2006). Diversity and biotechnological potential of the sponge-associated microbial consortia. J IndMicrobiolBiotechnol 33: 545–551.
- Wang,G, Sang-Hwal Yoon and Emilie Lefait (2009) Microbial communities associated with the invasive Hawaiian sponge Mycale armata The ISME Journal3, 374–377.
- Webster N, Hill RT (2001) Theculturable microbial community of the Great Barrier Reef sponge Rhopaloeidesodorabileis dominated by an a-Proteobacterium. Mar Biol 138:843–851.

This work is licensed under a Creative Commons Attribution 4.0 International License.





