Manuscript accepted on : 24 June 2012
Published online on: --
Samy A. Selim1, Sahar M. El Alfy1, Atef M. Diab2, Mohamed2, H. Abdel Aziz3and Mona S. Ewada3
1Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Al-Jouf University, Saudi Arabia. 2Department of Botany, Microbiology Section, Faculty of Science, Suez Canal University, Ismailia- 415 22, Egypt. 3Health Insurance Authority Hospital, Suez Governorate, Egypt.
ABSTRACT: The present work surveyed Haemodialysis (HD) units, in order to assess the bacteriological and toxicological qualities of dialysis water and dialysate. Also determine experimentally to how extent the bacterial activities are involved in immune depression for the patients. The most commonly isolated Gram-negative bacteria from treated water were Aeromonas spp. (22.2%). Endotoxin concentrations ranged between 3-113 (EU/mL) for water and dialysate respectively. The haematological analysis had non significant except haemoglobin level had a significant values (p = 0.02). The clinical data showed comparable difference than control patients. Missing, out of work, bypassed and even erroneously arranged treatment facilities are some of the reasons responsible for the detected high levels of bacterial and endotoxin in final product water. The presence and growth of bacteria in water and dialysate should be controlled and the endotoxin testing must be a part of the regular quality control regime, to minimize the risk of adverse reactions in HD patients.
KEYWORDS: Bacteria; endotoxins; haemodialysis patients; immune depression; Haemodialysis (HD)
Download this article as:| Copy the following to cite this article: Selim S. A, Alfy S. M. E, Diab A. M, Mohamed, Aziz H. A, Ewada M. S. Bacteriological and Toxicological Qualities of Dialysis Water and Associated Pyrogenic Reactions Involved in Haemodialysis Patients. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Selim S. A, Alfy S. M. E, Diab A. M, Mohamed, Aziz H. A, Ewada M. S. Bacteriological and Toxicological Qualities of Dialysis Water and Associated Pyrogenic Reactions Involved in Haemodialysis Patients. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9554 |
Introduction
Haemodialysis (HD) regime was introduced thirty years ago. Progress was permitted, but growing concerns about the inadequacy of tap water for dialysate production required, not only special water treatment system, but also very close monitoring program for the final product water quality of this system. Product water is prepared from municipal water passed through an on site water treatment facilities and distributed through polypropylene tubes to each dialysis unit in the center. Bacteria may be recognized in three main components in this procedure, water, dialysate and blood. There are many situations in which certain types of bacteria can persist actively, multiply, and produce high concentrations of endotoxin in the water, these problems are discovered when patients experience pyrogenic reactions from the endotoxin or septicemia from the bacteria itself. Patients with chronic renal failure are particularly susceptible to infections. Among factors predisposing to infection, impaired humeral and cell mediated immunity, nutritional deficiencies and iron overload are the most contributive (Boelaert et al., 1990; Goldman and Vanherwhen, 1990). Immune depression in renal failure patients is one of the serious complications that usually go worse with regular HD sessions. Contamination of dialysis water and dialysate with bacteria and bacterial toxins is regarded as the main cause of such complications. The involvement of bacteria and its pyrogenic reactions in this problem including symptomatic and asymptomatic baceriuria have been repeatedly recorded (Mako et al., 1985; Gordon et al., 1988; Watzke et al., 1989; Kulander et al., 1993; Bambauer et al., 1990; Phillips et al., 1994; Khedr et al., 1995; Laurence and Lapierre 1995; Matyus and Kakuk, 1995). In the absence of systemic infection, the most common exogenous cause of pyrogenic and/or septicemic reaction in HD patients is the transfer of bacteria, endotoxins or other pyrogens from contaminated dialysate (Bambauer et al., 1989) or
parenteral fluids (Kantor et al., 1983). Technical achievements in HD system contribute significantly to the reduction of the incidence of pyrogenic reactions.
The present work surveyed HD units in Suez Province, Egypt, in order to: 1. Assess the bacteriological and toxinological qualities of dialysis water and dialysate; 2. Evaluate the efficiency of treatment of the running systems in these centers to obtain data on HD water quality for the first time on a national basis, to assess the quality of HD water treated; 3. Proof the role of bacteria and bacterial toxins in wreaking the HD patient’s immunity and 4. Determine experimentally to how extent the bacterial activities are involved in immune depression for the patients.
Materials and Methods
Patients
All patients included in this study gave their informed consent. Patients with diabetes and recent infection episode were excluded. At the beginning of the study and after each period of treatment, two groups of data were evaluated in parallel: (i) haematological data: urea, haemoglobin, creatinine, serum albumin, haematocrit, adequate dialysis prescription and nutrition and (ii) clinical well-being data: Myolgia, hypotension, headache, itching, fever, Vomiting and chills. Blood samples were collected with 10 mL syringes from 159 HD patients in endotoxin free 10×75 mm glass tubes, and separated from native whole blood that been allowed to clot at room temperature for one hour, then centrifuged at 1100 g for 10 min after separation serum was transferred to disposable, endotoxin free, plastic Eppendorf tubes. Twenty random blood samples from normal persons were collected as control. The blood samples were collected at the same time, by the same technique and were prepared exactly like blood samples of HD patients serum or plasma.
Serological tests
Detection of immunological disorder percentage in the blood of HD patients carried out on blood samples depend on antibody-antigen reaction, where coagulation occurs as a result of the presence of antigens bodies (Gazenfeldt-Gazit and Eliahou, 1969). Streptolysin exotoxin which is produced by Streptococcus pyogenes (group A Streptococci) detected and neutralize by Anti Streptolysin-O-Titer (ASOT) kit which can exist of suspension contains polystyrenes fine particles coated with Streptolysin-O-toxin (HUMATEX, Germany). C-Reactive Protein (CRP) is abnormal alpha globulin exotoxin of Streptococcus pyogenes that appears rapidly in the serum of patients who have inflammatory condition. CRP kit consists of polystyrene fine particles coated with C-Reactive antibodies (HUMATEX, Germany). Widal tests stained Salmonella suspensions are used to detect, identify and quantitative specific antibodies in serum. These tests detect four type of salmonella antigens, STO (Salmonella Type O, somate antigen); STH (Salmonella Type H, flagellor antigen d); SPTA (Salmonella paratyphi -A, flageller antigen) and SPTB (Salmonella paratyphi –B, flageller antigen b) (Murex, England).
Sampling and microbiological examination of water and dialysate
Water samples from all the HD centers in Suez Province, Egypt were collected by our technically experienced personnel. In order to avoid any variability in sample examination all samples were analyzed in the same manner by the same people. Water samples collected during each clinic visit presented the inlet city water; after and the final product water and dialysate that feed HD units. A total of 84 water and 42 dialysate samples were investigated water samples were obtained from lines in continuous use. Duplicated samples were collected at the beginning of dialysis session with 10 mL syringes in endotoxin free, sterile 120 mL plastic containers. Dialysate of the bicarbonate type samples were collected directly from their original jerkins. In those containers of samples a sodium thiosulphate was added in a final concentration of 18 mg L–1, to neutralize any residual chlorine and prevent continuation of its bactericidal action during sample transit. Before sampling a solution of sodium hypochlorite (100 mg NaOCl/L) was applied to faucets and water let run for additional 2-3 min after treatment. Gloves and long sleeves were worn when collecting the samples to prevent skin bacteria from contaminating the samples. Microbiological examination was performed within 2 hr. During transportation sample containers were maintained at 4°C in portable coolers.
Quantitative methods were used to enumerate the total count of viable heterotrophic bacteria, total coliforms, faecal coliforms, faecal streptococci, Pseudomonas spp. and Aeromonas spp. The pour plate method was used to estimate the number of live heterotrophic bacteria. One millilitre, 0.1 and 0.01 ml volumes of each sample, in duplicate, were processed on standard plate count agar (Oxoid) by the pour plate techniques and incubated for 48 hr at 37°C. The membrane filter technique was employed for total coliforms, Pseudomonas spp. and Aeromonas spp. A volume of 100 ml of the samples were filtered through membrane filters with pores 0.45 µm in diameter. The membranes were then placed face up on m- Endo medium (Difco, 37°C, 24 h) for total coliforms, Cetrimide agar (Difco, 37°C, 24 h) for Pseudomonas spp., and Aeromonas selective media (Difco, 37°C, 24 h) for Aeromonas spp.
Pyrogen
For endotoxin determination both water and dialysate samples were immediately filtered through 0.2 µm sterile cellulose nitrate filters (MFS Cat. No. AO45 ho 47 A) to remove all microorganisms. Limulus
Amebocyte Lysate (LAL) tests were carried out, specimen dilution, endotoxin and lysate reconstitution, and preparation of positive and negative controls, initial lysate quality control and checking of label sensitivity were carefully followed as specified in (Sigma E-Toxicate LAL No. 210). Weekly exposure to endotoxin concentration for an ordinary HD patient, in average, was calculated depending upon the recommended dilution rate of water/dialysate, the clinical data about the volume of water/dialysate solution used in a session, flow rate and the number of the sessions weekly.
Statistical analysis
Categorical variables were analyzed by use of the x2 test or Fisher’s exact test. Continuous variables were analyzed by means of Student’s t test. Independent risk factors for colonization and acquisition were evaluated by means of a stepwise logistic regression model using variables that were statistically significant on crude analysis (p<0.05).
Results
Working status, heterotrophic bacteria counts (cfu/mL) of water and dialysate samples were recorded in Table 1. The mean values of the total heterotrophic bacteria counts and the percentage of samples exceeding the recommended standards.
The overall compliance of the tap water samples to our national standards was 100%, whereas the compliance of HD treated water and dialysate to AAMI standards was 85 and 97% respectively. The most commonly isolated Gram-negative bacteria from treated water were Aeromonas spp., 22.2%, followed by total coliforms (21.5.2%) and Pseudomonas spp. in samples (9.2%). All the centers showed almost the same bacterial diversity of gram -ve and +ve bacterial mpopulations. Contamination with endotoxins (EU/mL) in the final product water and dialysate samples was recorded in Table 1.
Table 1: Mean values of total heterotrophic bacteria, total coliforms, Pseudomonas spp. and Aeromonas (cfu/100 mL), endotoxins (EU/mL) in water and dialysate of haemodialysis centers.
| Tap water | Treated water | Dialysate | |
| Total heterotrophic bacteria | 197.8 | 234.2 | 101.0 |
| Total coliforms | 71.5 | 21.5 | 2.0 |
| Pseudomonas spp. | 37.3 | 9.2 | 14.4 |
| Aeromonas spp. | 56.2 | 22.2 | 9.7 |
| Endotoxins | 3.0 | 17.0 | 13.0 |
Readings ranged from 3-113 (EU/mL) for water and dialysate respectively. Regarding endotoxins concentration situation was presented to simplify the calculated endotoxin concentration for one ml of the water/dialysate mixture. In any dialyzer in center for example, endotoxin concentration in water was 3 EU/mL and in dialysate was 113 EU/mL that means a final concentration of 215 EU/mL for the water/dialysate mixture; as the mixture is usually made up of 34:1 water /dialysate. That means a concentration of 645×104 EU is introduced weekly to the patient on the basis that 30 L/h for four h/session is used in average for two sessions a week.
The base-line characteristics of HD patients were diagnosed are shown in Fig.1. The haematological analysis had non significant except haemoglobin level had a significant values (p = 0.02) than non HD patients. The clinical data (Myolgia, hypotension, headache, itching, fever, Vomiting and chills) showed comparable difference than control patients.
Detection of immunological disorder percentage in the blood of HD patients showed high level in HD patients. CRP recorded in 85% of patients where STH recorded the lowest level (17%) in patients.
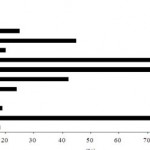 |
Figure 1: Percentage of some bacterial toxins specific antibodies and pyrogenic reactions in haemodialysis patients.
|
Discussion
In this study, in parallel with the total heterotrophic bacteria that colonize water-treatment systems, comprising the current recommendation according to the AAMI standards, we also investigated the counts of possible clinically significant bacteria as total coliforms, Pseudomonas spp and Aeromonas spp. The American Association of Medical Instrumentation (AAMI) recommends standards for treated water and dialysate quality. According to these standards the limits regarding the total heterotrophic bacteria counts are 200 colonies forming units/mL (cfu/mL) for treated water and 2000 cfu/mL for dialysate (AAMI, 1981). Clearly the results showed heavy loads of bacterial contamination in the final product water of all the studied centers, exceeding 200 cfu mL–1 the standard limits of the AAMI.
The bacterial counts were greatly higher than those detected in the inlet city water. This may indicate that the treatment facilities were sources of contamination, which is in line with what Bambauer et al. (1989; 1990) have reported. These high levels of bacterial contamination could be the absence of monitoring and maintenance programs associated with the aging systems and the unqualified labor that are in charge of the water treatment systems. Missing of some treatment facilities such as deionizers was also a reasonable cause of such a poor water quality. For the dialysate, 75% of the samples were found bacteriologically unacceptable for HD purposes according to the AAMI standards. The high bacterial counts in bicarbonate dialysate agreed with what Bambauer et al. (1990) described considering it as an ideal medium for growing bacteria. Harding et al. (1990) found that more than 35% of the centers studied had bacterial counts above the AAMI standards for both water and dialysate.
HD centers use water from a public supply originating from either surface or ground water, but in our country most public supply water is surface water. The source of the water supply may play an important role in bacteria and endotoxin content of the treated HD water, since surface waters usually contain endotoxin from Gram-negative bacteria and certain types of blue-green algae. Essentially, all water supplies are contaminated with ubiquitous water bacteria and consequently the water treatment, the distribution systems, and the dialysis machines are exposed repeatedly to continuous inoculation of these microorganisms. Even chlorinated water supplies commonly contain low levels of the water bacteria. We investigated the bacteriological quality of the mains water of the HD centers in order to assess any contamination of this water with bacteria that may influence the quality of treated water. According to our national standards, the total heterotrophic bacteria count in chlorinated drinking water should not exceed 50 cfu mL–1 at 37°C when incubated for 48 h, but it is not allowed to contain any total coliforms, Pseudomonas aeruginosa and Aeromonas spp. Percentage of gram positive bacteria was higher than gram negative, which may indicate the ability of water treatment facilities to get rid of gram negative bacteria more easily and efficiently than gram positive ones. This may interpretive the high concentration of endotoxins detected, as the endotoxins released when gram negative bacteria are killed or disintegrated. In addition the absence of final bacterial filtration just before introduction to dialysis sessions maximizes this problem. Calculations of the endotoxin concentration on weekly basis exposure stemmed from the risky phenomenon of shearing in which a large portion of the previously prepared antibodies of the HD patients is adhered to the dialysis membrane against the endotoxins on the other side of the dialysis membrane depending upon the filtrate flux events. Pillarella and Zydney (1988) proved some of the complications of this phenomenon. The sheared antibodies never return back again to the patient resulting in a gradual decrease in his immune responses.
Chills, fever, Hypertension, nausea and myalgia are sometimes present during HD and are commonly termed febrile or Pyrogenic Reactions (PRs). Such reactions in the absence of bacteremia are generally believed to be manifestation of bacterial endotoxemia. Endotoxins can cause PRs if introduce into the blood stream (Solano et al. 1975; Kantor et al., 1983). In investigation of PRs outbreak in a dialysis centers in Illinois the PRs were associated with reuse of dialyzes contaminated with endotoxins. Twenty two PRs occurred in 16 patients in 413 dialysis sessions more than half of these PRs cases were symptomatic, three cases required hospitalization but no deaths occurred and blood cultures were sterile. It is well established that the presence and growth of water bacteria should be prevented in HD water and dialysate (Favero et al., 1975) to minimize the risk of adverse reactions (Gazenfeldt and Eliahou, 1969), and endotoxemia (Tanguchi et al., 1990), in patients of chronic HD. To prevent pyrogenic reactions and bacteremia in HD patients the AAMI and CDC (Center of disease Control) recommend that, bacteriologic assay of HD fluids (water and dialysate) should be done at least monthly. In view of the available reports of endotoxic reactions in dialyzers (Solano et al., 1975; Kulander et al., 1993).
Conclusion
The presence and growth of bacteria in water and dialysate should be controlled and endotoxin tests must be part of the regular quality control regime to minimize the risk of adverse reactions in HD patients.
References
- Association for the Advancement of Medical Instrumentation (AAMI), 1981. American national standard for haemodialysis systems. ANSI/AAMI/RDS/81.
- Bambauer, R., G.A. Jutzler, and R. Schmidt, 1989. Ultrafiltration of dialysis fluid for haemodialysis. ASAIO Trans., 35: 516-530. PMID: 2597522
- Bambauer, R., J. Walther and W.K. Jung, 1990. Ultrafiltration of dialysis fluid to obtain a sterile solution during haemodialysis. Blood Purif., 8: 309-317. PMID: 2093329
- Boelaert, J.R., B.F. Cantinieaux, C. Harringa and P.G. Fondu, 1990. Recombinant eruthropoietin reverses PMNLs granulocytes dysfunction in iron overloaded dialysis patients. Nephrol Dial Transplant, 5: 504-507.PMID: 2130296
- Favero, M.S., N.J. Petersen, L.A. Carson, W.W. Bond and S.H. Hindman, 1975. Gram negative water bacteria in haemodialysis systems. Health Lab. Sci., 12: 321-334. PMID: 1236620
- Gazenfeldt-Gazit, E. and H.E. Eliahou, 1969. Endotoxin antibodies in patients on haemodialysis. Israel J. Med. Sci., 5: 1032-1036. PMID: 4984050
- Goldman, M. and J.L. Vanherwhen, 1990. Bacterial infections in chronic hemodialysis patients epidemiologic and pathophysiologic aspects. Adv. Nephrol., 19: 315-332. PMID: 2105586
- Gordon, S.M., M. Tipple, L.A. Bland and W.R. Jarvis, 1988. Pyrogenic reactions associated with the reuse of disposable hollow fiber hemodialyzers. J. Am. Med. Assoc., 260: 2077-2081. PMID: 3418872
- Harding, G.B., E. Klein, T. Pass, R. Wright and C. Million, 1990. Endotoxin and bacterial contamination of dialysis center water and dialysate: A cross sectional survey. Int. J. Artif Organs., 13: 39-43. PMID: 2394493
- Kantor, J.R., L.A. Carson and D.R. Graham, 1983. Outbreak of pyrogenic reactions at a dialysis centers associated with infusion heparinaized saline solution. Am. J. Med., 74: 449-456. PMID: 6829590
- Khedr, M.S., A.A. Abdelrahman and A.M. Diab, 1995. Asymptomatic bacteriuria in chronic haemodialysis patients. Egy. J. Med. Microbiol 4: 162-167.
- Kulander, L., U. Nisbeth, B.G. Danielsson and O.E. Eriksson, 1993. Occurrence of endotoxin in dialysis fluid from 39 dialysis units. J. Hosp Infect., 24: 29-37. PMID: 8101200
- Laurence, R.A. and S.T. Lapierre, 1995. Quality of haemodialysis water: A 7- year multicenter study. Am. J. Kidney Dis., 25: 738-750. PMID: 7747728
- Mako, J., J. Jansen, B. Bognar and A. Farago, 1985. Purulent pericarditis caused by Staphylococcus aureus in two patients undergoing hemodialysis. Int. J. Urol Nephrol., 17: 79-83. PMID: 3997409
- Matyus, J. and G. Kakuk, 1995. Amyloidosis associated with dialysis. Orv. Hetil, 136: 587-593.
- Phillips, G., S. Hudson and W.K. Steuart, 1994. Persistence of microflora in biofilm within fluid pathways of contemporary hemodialysis monitors, (Gambro AK 10). J. Hosp Infect, 27: 117-125. PMID: 7930538
- Pillarella, M.R. and A. Zydney, 1988. Analysis of solute clearance and flux in pre and post dilution hemofiltration. ASAIO-Trans., 34: 415-419. PMID: 3196540
- Solano, J.T., L.B. Schonberger and N.J. Petersen, 1975. Pyrogenic reactions during hemodialysis caused by extramural endotoxin. Lancet, 2: 732-734.PMID: 52769
- Tanguchi, T., S. Katsuhiema and K. Lee, 1990. Endotoxemia in patients on hemodialysis. Nephron, 56: 44-49. http://content.karger.com/ProdukteDB/produkte.asp?doi=186099
- Watzke, H., G. Mayer, H.P. Schwarz, G. Stanek and M.L. Rotter et al., 1989. Bacterial contamination of dialysate in dialysis-associated endotoxaemia. J. Hosp Infect., 13: 109-115. PMID: 2567302

This work is licensed under a Creative Commons Attribution 4.0 International License.





