Manuscript accepted on : 22 October 2011
Published online on: 28-12-2011
Mohammad Sadeghi1*, Nahid Ghasemi¹ and Mojgan Yarahmadi2
¹Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak Iran.
²Department of English, Faculty of Humanities, Arak Branch, Islamic Azad University, Arak Iran.
Corresponding author E-mail: m-sadeghi@iau-arak.ac.ir
ABSTRACT: Biosuperabsorbent polymers are obtained by the graft copolymerization of acrylic acid (AcA) and acrylonitrile(AN) monomers onto CMC, using Ceric ammonium nitrate as a free radical initiator in the presence of methylene bisacrylamide as a crosslinker. Graft Evidence of grafting was obtained by comparison of FTIR spectra of CMC and homopolymer-free H-CMC-g-poly(AcA-co-AN). The effect of the concentration of the initiator, monomers, polysaccharide and crosslinker as well as chemical-invironment parameters on the swelling amount of biosuperabsorbent were investigated to achieve the optimum absorbency.
KEYWORDS: CMC; hydrogel; Ceric ammonium nitrate; acrylic monomers; optimization
Download this article as:| Copy the following to cite this article: Sadeghi M, Ghasemi N, Yarahmadi M. Investigation of Effective Parameters in Synthesis of Biosuperabsorbent Hydrogel based on Carboxymethylcellulose. Biosci Biotechnol Res Asia 2011;8(2) |
| Copy the following to cite this URL: Sadeghi M, Ghasemi N, Yarahmadi M. Investigation of Effective Parameters in Synthesis of Biosuperabsorbent Hydrogel based on Carboxymethylcellulose. Biosci Biotechnol Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9200 |
Introduction
The market for superabsorbent polymers (SAPs) has increased by a factor of 5 over the past 10 years. These materials are crosslinked hydrophilic polymers, capable of absorbing large quantities of water, saline or physiological solutions.1,2 The absorbed fluids are hardly removable even under some pressure. They are widely used in various applications such as hygienic, foods, cosmetics, and agriculture.2-4 This accounts for increase in the worldwide production of superabsorbent polymers (SAPs) from 6000 tons in 1983 to 450000 tons in 1996.1 Nowadays, the worldwide production of SAPs is more than one million tons in year. Hence, synthesis and investigation of specific and new superabsorbent hydrogels with high absorbency, mechanical strength and initial absorption rate, is the main goal of the several research groups in the world.5-10.
The properties of the swelling medium (e.g. pH, ionic strength and the counter ion and its valency) affect the swelling characteristics. SAPs responding to external stimuli such as heat, pH, electric field, chemical environments, etc, are often referred to as “intelligent” or “smart” polymers. Among these, pH-sensitive hydrogels have been extensively investigated for potential use in site-specific delivery of drugs to specific regions of the gastrointestinal tract and have been prepared for delivery of low molecular weight protein drugs. Therefore, these hydrogels have important applications in the field of medicine, pharmacy, and biotechnology.11,12
Natural-based superabsorbent hydrogels have attracted much interest from the viewpoint of improving the tissue tolerance of synthetic polymers and the mechanical properties of natural polymers. The presence of the natural parts guarantees biodegradability of the superabsorbing materials. Because of their biocompatibility, biodegradability and non-toxicity, natural polymers, i.e. polysaccharides and proteins, are the main part of these biopolymers. One of the best methods for the synthesis of these superabsorbent hydrogels is graft copolymerization of vinylic monomers onto natural polymers. Monomers such as acrylonitrile (AN), acrylic acid (AA), acrylamide (AAm) have been graft copolymerized onto polysaccharides such as starch, cellulose and their derivatives.13-15 The first industrial superabsorbent hydrogel was synthesized using this method via ceric-induced graft copolymerization of acrylonitrile onto starch followed by alkaline hydrolysis of the resulted graft copolymer.16
According to the literature survey based on Chemical Abstract Service, a few studies have been reported in the case of polysaccharide-based hydrogels.17-19 Hence, the objective of the present paper is to describe the preparation and optimized effective parameters of a CMC-g-poly(acrylic acid-co-acrylonitriled) hydrogel as a new natural-based polymer with high swelling capacity.
Experimental
Materials
The polysaccharide, CMC (chemical grade, MW 50000) was purchased from Merck Chemical Co. (Germany). Acrylic acid(Merck, Darmstadt, Germany), Acrylonitrile (Merck, Darmstadt, Germany), were used after recrystalization and distillation for removing inhibitors, respectively. N‘,N‘-methylene bisacrylamide and Ceric ammonium Nitrate (Fluka, Buchs, Switzerland) were of analytical grade and used without further purification. Double distilled water was used for the hydrogel preparation and swelling measurements.
Preparation of hydrogel
A pre-weighed amount of CMC (0.5-1.5 g) was dissolved in 40 mL degassed distilled water in 50 mL three-neck reactor equipped with a mechanical stirrer (RZR 2021, a three-blade propeller type, Heidolph, Schwabach, Germany) and the reactor was immersed in a thermostated water bath preset at a desired temperature (65oC). Then acrylic acid (1.0-3.0 g) and acrylonitrile (1.0-3.0 g) were added simultaneously to the reactor. After stirring for 10 min, Ceric ammonium Nitrate (0.01-0.40 g CAN in 5 mL H2O-HNO3) and methylene bisacrylamide (0.05-0.20 g in 5 mL H2O) were added simultaneously to the reaction mixture. The temperature was maintained at 65 oC and the reaction mixture was stirred continuously (300 rpm) for 1 h. At the end of the propagation reaction, the gel product was poured into ethanol (200 mL) and was dewatered for 12 h. Then, the product was cut into small pieces, washed with 200 mL ethanol and filtered. The particles were dried in an oven at 50 oC for 12 h. After grinding, the powdered superabsorbent hydrogel was stored in absence of moisture, heat and light.
Swelling measurements
An accurately weighed sample (0.2 ± 0.001 g) of the powdered superabsorbent with average particle sizes between 40-60 mesh (250–350) was immersed in distilled water (200 mL) and allowed to soak for 3 h at room temperature. The equilibrium swelling (ES) capacity was measured twice at room temperature according to a conventional tea bag (i.e. a 100 mesh nylon screen) method and using the following formula:
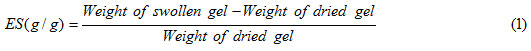
Swelling in various salt solutions
Absorbency of the optimized sample was evaluated in 0.15 M solutions of NaCl, CaCl2, and AlCl3 according to the earlier method described for swelling measurement in distilled water.
Results and Discussion
spectral characterization of hydrogel
FTIR spectroscopy was used for identification of the hydrogel. Figure 1 shows the IR spectra of the CMC and the resulted hydrogel. The band observed at 1607 cm-1 , 1430 cm-1 and 1330 cm-1 be attributed to C=O stretching and bending in carboxylate(COO –) functional groups of substrate backbone (Figure 1a). The broad band at 2500-3500 cm-1 is due to stretching of –OH groups of the CMC. In the spectra of the hydrogel the characteristic bands at 1637 cm-1 , 1715 cm-1 and 2240 cm-1 were attributed to carboxyamide( MBA crosslinker) , carboxylic acid (AcA monomer) and cyanide groups (acrylonitrile monomers) stretching respectively.
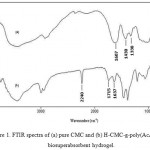 |
Figure 1: FTIR spectra of (a) pure CMC and (b) H-CMC-g-poly(AcA-co-AN) biosuperabsorbent hydrogel.
|
To obtain additional evidence of grafting, a similar polymerization was conducted in the absence of the crosslinker. After extracting the homopolymers, PAcA or PAN and unreacted monomers using a cellophane membrane dialysis bag (D9402, Sigma–Aldrich), an appreciable amount of grafted CMC (84%) was observed. The graft copolymer spectrum was very similar to Figure 1b. Also according to preliminary measurements, the sol (soluble) content of the hydrogel networks was as little as 1.9 %. This fact practically proves that all AcA and AN are involved in the polymer network. So, the monomers percent in the network will be very similar to that of the initial feed of reaction.
Optimization of the grafting variables
In this work, the main factors affecting on the grafting conditions, i.e. concentration of CMC, MBA, AN/AcA weight ratio, CAN, were systematically optimized to achieve biosuperabsorbent with maximum water absorbency. Finally, the chemical-invironment factors affecting on the absorbency amount were investigated.
Effect of polysaccharide concentration
The effect of CMC weight on hydrogel swelling is shown in Figure 2. Maximum swelling (283.6 g/g) has been observed at 1.0 g of CMC, while other factors including monomers, initiator, and MBA concentration were kept constant. Swelling capacity increased by increasing the CMC weight from 0.25 to 1.5 g from 98.5 to 157.1 g/g. As the CMC weight was increased in the polymerization feed, the active sites can react easily with monomers. Increasing CMC content more than 1.0 g, results in a high viscosity of the medium and a decrease in the diffusion of monomers to active sites to produce crosslinked hydrogels14.
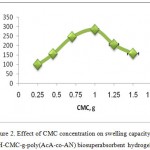 |
Figure 2: Effect of CMC concentration on swelling capacity of H-CMC-g-poly(AcA-co-AN) biosuperabsorbent hydrogel.
|
Effect of MBA concentration
The effect of crosslinker concentration on swelling capacity of H-CMC-g-poly(AcA-co-AN) was investigated. As shown in Figure 3, more values of absorbency are obtained by lower MBA concentration as reported by pioneering scientists.8,19 In fact, higher crosslinker concentrations decrease the free space between the copolymer chains and consequently the resulted highly crosslinked rigid structure can not be expanded and hold a large quantity of water. The maximum absorbency (400.9 g/g) is achieved at 0.0108 mol/L of MBA.
 |
Figure 3: Effect of MBA concentration on swelling capacity of H-CMC-g-poly(AcA-co-AN) biosuperabsorbent hydrogel. |
Effect of AcA/AN Weight Ratio on the swelling capacity
Different superabsorbent hydrogels with various AcA/AN percent weight were synthesized by changing the amount of PAcA (0.50-1.50 g) and PAN (0.50-1.50 g). The maximum water absorbency (275.3.0 g/g) was obtained at AN/AcA=0.7 (percent weight of AN/AcA=0.7 (Fig.4). The initial increase in water absorbency could be originated from the greater availability of monomer molecules in the vicinity of the chain propagating sites of CMC macroradicals. In addition, higher AA content enhances the hydrophilicity of the hydrogel that, in turn, causes a stronger affinity for more absorption of water. Indeed in high concentration of charged ionic groups existing in copolymer chains along with increase of NaAA in the hydrogel increases the swelling capacity due to osmosis and charge repulsion17. In other words, the presence of more ionic groups in the polymer chains results in increased swelling, because the ionic groups are more strongly solvated than non-ionic groups in the aqueous medium. Similar conclusions were reported by other investigators.
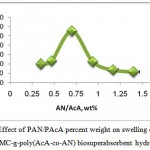 |
Figure 4: Effect of PAN/PAcA percent weight on swelling capacity of H-CMC-g-poly(AcA-co-AN) biosuperabsorbent hydrogel.
|
Effect of initiator concentration
The effect of the CAN concentration on the swelling capacity of the hydrogels is given in Figure 5. The maximum swelling was obtained at initiator amount of 0.009 mol/L. the swelling of hydrogel increased initially on increasing the initiator concentration up to 0.009 mol/L. the increase of swelling may be ascribed to the increase of the active sites on the on the backbone of the CMC arising from the attack of Ce4+, and so more hydrophilic AcA/AN ratio grafting takes place18. The swelling decreased with the increase of the initiator concentration beyond the optimum condition. This behavior can be attributed to: (a) oxidative degradation of CMC chains by excess Ce4+ ions, (b) an increase in the termination reaction of the chain radicals via bimolecular collision because of an increased population of macroradicals produced, and (c) enhancement in homopolymerization reaction. These observations are in agreement with similar observations reported by others 18,19.
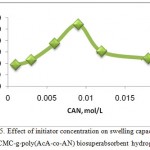 |
Figure 5: Effect of initiator concentration on swelling capacity of H-CMC-g-poly(AcA-co-AN) biosuperabsorbent hydrogel. |
Swelling in various salt solutions
Swelling capacity in salt solutions is of prime significance in many practical applications such as personal hygiene products and water release systems in agriculture. The swelling ability of “anionic” hydrogels in various salt solutions is appreciably decreased compared to the swelling values in distilled water. This well-known undesired swelling-loss is often attributed to a “charge screening effect” of the additional cations which causing a non-perfect anion–anion electrostatic repulsion.29 Also, in salt solution the osmotic pressure resulting from the difference in the mobile ion concentration between gel and the aqueous phases is decreased and consequently the absorbency amounts are diminished. In addition, in the case of salt solutions with multivalent cations, “ionic crosslinking” at surface of hydrogel particles causing an appreciably decrease in swelling capacity 3,9.
In this series of experiments, the swelling capacity was measured in various salt solutions. The effect of charge of cation on swelling can be concluded from Figure 6. With increasing the charge of cation, degree of crosslinking is increased and swelling is consequently decreased. Therefore, the absorbency of the synthesized hydrogel is in the order of NaCl>CaCl2>AlCl3.
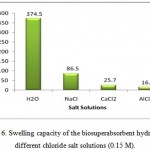 |
Figure 6: Swelling capacity of the biosuperabsorbent hydrogel in different chloride salt solutions (0.15 M). |
As given in Figure 6, the swelling capacity of the hydrogels in CaCl2 solution is lower than that of in NaCl solutions. As mentioned above, in the presence of the bivalent calcium ions, the crosslinking density increases because of a double interaction of CaCl2 with sulfate groups leading to ionic crosslinking. When this hydrogel is immersed in calcium chloride solution, it deswells to a collapsed form. When the shrinked hydrogel is immersed in sodium chloride solution again, the calcium ions are replaced by sodium ions. This ion exchange disrupts the ionic crosslinks leading to swelling enhancement. As a result, when hydrogel is treated alternatively with NaCl and CaCl2 solutions with equal molarity, the swelling reversibility of hydrogel is observed. This chemical behavior of hydrogel is resulted from the ion exchange ability of the sulfate groups 18.
Conclusion
In following synthesis of a novel biosuperabsorbent hydrogel was via graft copolymerization of acrylic acid and acrylonitrile onto CMC in the presence of a crosslinking agent, we optimized reaction conditions of polymerization to obtain maximum water absorbency (286 g/g) and they were found to be: CMC 1.0 g, MBA 0.0324 mol/L, CAN 0.009 mol/L and percent weight of AN/AcA 0.7. Swelling measurement in various salt solutions showed that the synthesized hydrogels are low salt-sensitive. However, swelling-loss in salt solutions, in comparison with distilled water, can be attributed to charge screening effect and ionic crosslinking for mono- and multi-valent cations, respectively. The swelling capacity in CaCl2 is much lower than that in NaCl solution and distillated water.
References
- Buchholz, F. L.; Graham, A. T. In Modern Superabsorbent Polymer Technology; New York: Wiley, (1997).
- Peppas, L. B.; Harland, R. S. In Absorbent Polymer Technology; Amsterdam: Elsevier, (1990).
- Loh, X. J.; Peh, P.; Liao, S.; Sng, C.; Li, J. J Control Release, 143, 175-182(2010).
- Liu, Y.; Chan-Park, M. Biomaterials, 30, 196-207(2009).
- Rasool, N.; Yasin, T.; Heng, J.; Akhter, Z. Polymer, 51, 1687-1693(2010).
- Tanuma, H.; Saito, T.; Nishikawa, K.; Dong, T.; Yazawa, K.; Inoue, Y. Carbohyd Polym, 80, 260-265(2010).
- Pourjavadi, A.; Harzandi, A. M.; Hosseinzadeh, H. Eur Polym J, 40, 1363-1371(2004).
- Tang, Q.; Lin, J.; Wu, J.; Zhang, C.; Hao, S. Carbohyd Polym, 67, 332-336(2007).
- Sugahara, Y.; Takahisa, O. J Appl Polym Sci, 82, 1437-1443(2001).
- Mi, P.; Ju, X.; Xie, R.; Wu, H.; Ma, J.; Chu, L. Polymer,51, 1648-1653(2010).
- George, M.; Abraham, T. E. Int J Pharma, 335, 123-129(2007).
- Sadeghi, M.; Hosseinzadeh, H. Turk J Chem, 32, 375-388(2008).
- Bajpai, A. K.; Giri, A. Carbohydr Polym,53, 271-278(2003).
- Mahdavinia, G. R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M. J. Europ Polym J, 40, 1399-1407(2004).
- Fanta, G. F. In Polymeric Materials Encyclopedia. Salamone, J. C., editor. Florida: CRC Press, Boca Raton, vol.10. p. 7901, 8051(1996).
- Rathna, G.V.N.; Damodaran, S. J Appl Polym Sci, 85, 45-51(2002).
- Yan, H.; Saiani, A.; Gough, J. E.; Miller, A. F. Biomacromolecules, 7, 2776-2782(2006).
- Pourjavadi, A.; Kurdtabar, M.; Mahdavinia, G. R.; Hosseinzadeh, H. Polym Bull, 57, 813-824(2006).
- Sadeghi,M, Yarahmadi,M, Oriental Journal of Chemistry, vol.27, no.2, 453-460,(2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.





