Manuscript accepted on : September 03, 2011
Published online on: --
K. V. Rajesh¹*, P. Jawahar Babu¹, G. Giri Prasad¹, T. Sudheer¹ and G. Sudheer¹
Department of Biotechnology, Bapatla Engineering College, Andhra Pradesh - 522 101 India.
ABSTRACT: In recent years there has been a phenomenal increase in the use of alkaline proteases as industrial catalysts. These enzymes offer advantages over the use of conventional chemical catalysts for numerous reasons, for example they exhibit high catalytic activity, a high degree of substrate specificity, can be produced in large amounts and are economically viable. Bacterial alkaline proteases constitute an important group of industrial enzymes. The objective of the present study was to determine the significant parameters in the economical production and optimize the conditions for alkaline protease by Bacillus subtilis MTCC1790 in submerged fermentation by using Taguchi experimental design. This approach facilitated the study of interaction of a large number of variables spanned by factors and their settings with a small number of experimental runs leading to considerable economy in time and cost for the process optimization. There are Five factors viz., carbon, nitrogen, metal ions, inoculum% and agitation, each at two levels were selected and an orthogonal array layout of L16 (4)5 performed. The experiment result indicated that dextrose (0.6%), NH4Cl (0.6 %), Ca2+ as metal ions, inoculum (8%) and agitation (100 rpm) were the important factors for alkaline protease production. At this optimum condition, the yield of protease production by Bacillus subtilis MTCC1790 was found to be 286 U/mL.
KEYWORDS: Bacillus subtilis MTCC1790; Alkaline Protease; Taguchi design; Submerged fermentation
Download this article as:| Copy the following to cite this article: Rajesh K. V, Babu P. J, Prasad G. G, Sudheer T, Sudheer G. Economical Production and Optimization of Alkaline Protease from Bacillus Subtilis MTCC1790 by using Taguchi Design. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Rajesh K. V, Babu P. J, Prasad G. G, Sudheer T, Sudheer G. Economical Production and Optimization of Alkaline Protease from Bacillus Subtilis MTCC1790 by using Taguchi Design. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9489/ |
Introduction
Proteases, the most important group of industrial enzymes constitute nearly 40% of the total worldwide enzyme sales in various industrial market sectors, such as detergent, food and pharmaceutical, leather, silk and for recovery of silver from used x-ray films7. Alkaline proteases are produced by a wide range of microorganisms including bacteria, fungi, yeast, actinomycetes and also mammalian tissues. Among bacteria, Bacillus sp. is specific producers of alkaline proteases2. These are used as cleansing additives in detergents to facilitate the release of proteinaceous materials in stains due to grime, blood, milk, etc. Bacillus sp. grows in a pH range of 7.0 – 11.0 and produces extra cellular alkaline proteases8.The increased usage of the protease as a detergent additive is mainly due to its environmentally acceptable cleaning capabilities.In view of the promising applicability of Alkaline proteases in detergents, pharmaceutical, brewery, leather, food and agricultural industries it should be produced in high yields in a low-cost medium with high purity and stability. In biotechnology based industrial processes, the formulation of fermentation medium is of critical importance for enhancing the product concentration, yield and volumetric productivity9. Conventional fermentation media optimization studies apply single factor optimization, which is done by maintaining all the variables unchanged at a time. This method is time consuming and requires large number of experiments in order to determine the optimal level of each factor.
Alternative to conventional optimization procedures, design of experiments (DOE) and statistical tools help to gain more information about the optimization conditions in a few trials (1, 3). Among various statistical experimental designs, Taguchi experimental design offers distinct advantages by which many factors can be examined simultaneously and much quantitative information can be extracted with a few experimental trials4. Taguchi constructed a special set of general design guidelines for factorial experiments that cover many applications. The method uses a special set of arrays called orthogonal arrays, which stipulate the way of conducting the minimal number of experiments and give the full information of all the factors that affect the performance parameter5-6. While there are many standard orthogonal arrays available, each of the arrays is meant for a specific number of independent design variables and levels. The additive assumption of the Taguchi design implies that the individual or main effects of the independent variables on performance parameter are separable10. Under this assumption, the effect of each factor can be linear, quadratic or may have higher order, but the model assumes the absence of any cross-product effects (interactions) among the individual factors. That means the effect of independent variable on performance parameter does not depend on the different level settings of any other independent variables and vice versa11-12. The crux of the orthogonal arrays method lies in choosing the level combinations of the input design variables for each experiment.
To the best of our knowledge the nutritional and environmental conditions for submerged culture of Bacillus SubtilisMTCC1790 for Alkaline proteases production using orthogonal matrix has not been demonstrated. In the present study, Taguchi method was applied to test the relative importance of medium components (i.e., carbon, nitrogen and metal ions) and environmental factors (i.e., temperature and agitation speed) for economical production of alkaline protease.
Material and Methods
Bacterial Strain
The bacterial strain used throughout the study for the production of alkaline proteases was Bacillus SubtilisMTCC1790.It was purchased from Institute of Microbial Technology (IMTECH), Chandigarh.
Revival and Maintenance of the Culture Media
The culture was revived as per the standard procedure. The revival medium was prepared with the following composition per litre is beef extract 1g, yeast extract 2g ,Peptone 5g, NaCl 5g, Agar 20g. The pH of the medium was adjusted to 7.0 with 1N NaOH or 1N HCl and was autoclaved at 121°C for 15 minutes. The suspension of the strain was streaked on to petriplates having media of the above composition. From the plates, single colonies were streaked on to culture tubes. After incubation for 72 hours at 37°C, the well grown slants were kept in the refrigerator. The medium prepared for the production of protease from Bacillus subtilisMTCC1790 medium containing (g/l) contain glucose 10g, peptone5g, yeast extract 5g, KH2PO4 1g, MgSO4 0.2g. The pH of the medium was adjusted to 10.0 with 1N NaOH or 1 N HCl and was autoclaved at 121°C for 15 minutes.
Production of proteases
100 ml of nutrient broth was inoculated with 1 ml of the inoculum (containing 106cells/ml) and was incubated at 30°C for 18 hours at 120rpm. After incubation the crude enzyme was obtained by centrifugation of the culture broth at 10000×g for 10 minutes at 30°C. The cell free supernatant which contains the enzyme was assayed for alkaline protease activity.
Growth curve
Growth pattern of selected Bacillus Subtilisstrain was determined by incubating at 30ºC, 120rpm for 18h in above mentioned fermentation medium. Samples were drawn at the interval of 2h to determine cell growth and enzyme activity. Cell growth was monitored turbidimetrically by spectrophotometer at 595nm.
Enzyme source
The culture filtrate taken at regular intervals of 24 hours served as the crude enzyme source. 5 ml supernatant was taken aseptically from each flask, cooled in ice, and centrifuged at 10000 rpm for 10 minutes. The supernatant served as the source of crude enzyme.
Enzyme assay
Alkaline Protease assay was done by a modification of the casein digestion method of Kunitz (1947). To 3 ml of 0.6% casein in a phosphate buffer (100 mM) of pH 7, 0.5 ml of crude enzyme was added and incubated for 30 minutes at 37°C. After which 3 ml of 5% trichloroacetic acid was added to stop the reaction and allowed to stand for 15 minutes at room temperature. The resultant mixture was filtered through Whatman No. 1 filter paper. The absorbance of this filtrate was measured at 280 nm in a UV Visible spectrophotometer. A suitable control was run simultaneously, in which Trichloroacetic acid (TCA) was added prior to the addition of enzyme solution. One unit of proteolytic activity was defined as that amount of enzyme, which liberated 1µg of tyrosine per ml per minute under the specific conditions of assay. The absorbance at 280 nm (test-control) indicated the tyrosine content of the filtrate, which has been released by the hydrolysis of the protein substrate by the enzyme. The tyrosine content of the sample was read from the standard calibration curve prepared with pure tyrosine.
Enzyme Production Optimization
The production of alkaline protease substituting with carbon sources (1%w/v) of dextrose, sucrose, fructose, lactose, mannitol, maltose and starch were evaluated. Five different organic nitrogen sources (1%w/v) viz.Yeast extract (0.5%w/v) and peptone (0.5%w/v), peptone, casein, (NH4)2SO4, NH4NO3, (NH4) H2PO4, NaNO3, NH4Cl were evaluated. The effect of incubation time 0 to 36 h, temperature 35 to 500c and pH 8-11.5 of the growth medium were studied.
Taguchi design
Taguchi orthogonal array design plans the minimal number of experiments and supply outright information for all the factors. According to the results obtained, best carbon and nitrogen sources were selected along with metal ions, inoculum and agitation. These parameters were optimized using L16 orthogonal array design with help of MINITAB 16 software.
Results
Optimization of alkaline protease production from Bacillus SubtilisMTCC1790 Screening of important Carbon and Nitrogen sources Selection of most suitable carbon and nitrogen sources by one-variable-at-a-time approach .The Bacillus sp. capable of utilizing wide range of carbon sources. However the best carbon source in present study of alkaline protease production was dextrose followed by fructose shown in fig 1.In general both inorganic and organic nitrogen sources were utilized efficiently by Bacillus sp. for alkaline protease production. However inorganic source ammonium chloride gives better results followed by yeast extract and peptone for enzyme production illustrated in Fig.2.
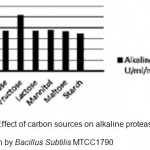 |
Figure 1: Effect of carbon sources on alkaline protease production by Bacillus SubtilisMTCC1790. |
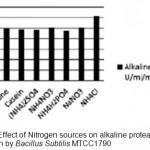 |
Figure 2: Effect of Nitrogen sources on alkaline protease production by Bacillus SubtilisMTCC1790. |
Screening of important physico-chemical determinants
Alkaline protease produced throughout the course of fermentation is 4-36 h, the enzyme production was high at 20th hour and corresponded with late exponential phase of growth shown fig 3. Ward 1980 has also reported that Bacillus SubtilisMTCC1790 usually produces more alkaline protease during late exponential phase.
The function of this enzyme is obscure, but its production is correlated with outset of high rate of protein turnover during sporulation in certain Bacilli Subtilisdepicts that maximum protease production. In present study, the maximum alkaline protease activity achieved for Bacillus Subtilis MTCC1790 was at intial pH 10.5 and temperature at 40°C are shown in Fig.4.
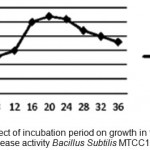 |
Figure 3: Effect of incubation period on growth in terms of Alkaline Protease activity Bacillus SubtilisMTCC1790.
|
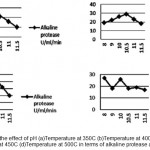 |
Figure 4: Shows the effect of pH (a)Temperature at 350C (b)Temperature at 400C (c) Temperature at 450C (d)Temperature at 500C in terms of alkaline protease activity. |
Optimization of the key determinants by L16 (4)5 Orthogonal array Design
Taguchi experimental design is a good positive option for the optimization of biotechnological processes for production of microbial enzymes. In this case, the influence of five factors on the protease production by Bacillus SubtilisMTCC1790 was tested in Taguchi experimental design in 16 runs with four levels and five parameters i.e. carbon, nitrogen, metal ions, inoculum and agitation selected for optimization of alkaline protease production represented in table.1. Alkaline protease production from Bacillus SubtilisMTCC1790 shows the efficiency in ranging from 33.41 – 286.81U/mL corresponding to the combined effect of the five factors in their specific ranges (Table2). The experimental results suggest that these factors at optimum level strongly support the production of protease.
In run 11, dextrose (0.8%), NH4Cl (0.6%), Mn2+, inoculum (6%) and agitation (0 rpm) have lowest enzyme activity of 33.41U/mL was observed. Where as in run2, with a combination of Dextrose (0.6%), NH4Cl (0.6 %), Ca2+, inoculum (8%) and agitation (100 rpm) was achieved activity of 286.81U/mL (Table 2). For studying the main effect of each factor, the data were analysed by using MINITAB 16.
Table 2: L16 (45) orthogonal array of Taguchi experimental design and corresponding alkaline protease production by Bacillussubtilis MTCC1790.
| Runs | Dextrose | NH4Cl | Metal | Inoculum | Agitation | Enzyme |
| (%) | (%) | ions | (%) | (rpm) | activity U/ml | |
| 1 | 3 | 4 | 2 | 1 | 3 | 96.2 |
| 2 | 2 | 2 | 1 | 4 | 3 | 286.81 |
| 3 | 1 | 1 | 1 | 1 | 1 | 112.16 |
| 4 | 4 | 1 | 4 | 2 | 3 | 31.81 |
| 5 | 3 | 1 | 3 | 4 | 2 | 149.54 |
| 6 | 4 | 4 | 1 | 3 | 2 | 98.53 |
| 7 | 1 | 2 | 2 | 2 | 2 | 77.61 |
| 8 | 4 | 3 | 2 | 4 | 1 | 180.52 |
| 9 | 2 | 4 | 3 | 2 | 1 | 106.68 |
| 10 | 1 | 4 | 4 | 4 | 4 | 137.79 |
| 11 | 3 | 2 | 4 | 3 | 1 | 33.41 |
| 12 | 3 | 3 | 1 | 2 | 4 | 176.62 |
| 13 | 2 | 3 | 4 | 1 | 2 | 165.60 |
| 14 | 1 | 3 | 3 | 3 | 3 | 153.02 |
| 15 | 2 | 1 | 2 | 3 | 4 | 117.68 |
| 16 | 4 | 2 | 3 | 1 | 4 | 86.41 |
Discussion
In this study, alkaline protease production by Bacillus subtilisMTCC1790 in submerged fermentation, Dextrose (0.6 %) as carbon source, (NH4)Cl (0.6 %) as nitrogen source, Ca2+ as metal ion, inoculum (8%) and agitation (100 rpm) was at level 2, level 2, level 1, level 4 and level 3 respectively constituted the main factors of the medium.Taguchi design was successfully applied to test the relative importance of medium components and environmental factors on alkaline protease production. The response table for means and S/N ratio (signal-to-noise) larger is better are computed and summarized in Table 3. Rank and delta values shown in the last two rows in the tables assist in estimating the effect of factors. Delta measures the extent of the effect by calculating the difference between the highest and lowest data mean for a factor. The higher the delta value, the greater the suggested effect of that component. Rank arranges the factors to form the greatest effect to the least effect on the basis of the delta values. As presented in Table 3, it can be observed that inoculum, metal ions contributing have shown highest positive impact on the alkaline protease production and agitation showed least impact among the factors studied with the assigned variance of values. Figure .5 shows the main effect plot for the mean and for the S/N ratio. MINITAB 16 creates the main effect plot by plotting the data mean for each factor level. These means are the same as those shown in Table 3. Lines connect the point for each factor. There is no main effect present when the line is flat. However, when the line is not flat, then there is a main effect present.
Table 3(a): Shows the response tables for Mean and Signal to Noise Ratios.
| Level | Dextrose | NH4Cl | Metal | Inoculum | Agitation |
| (%) | (%) | ions | (%) | (rpm) | |
| 1 | 120.15 | 102.80 | 168.53 | 115.09 | 108.19 |
| 2 | 169.19 | 121.06 | 118.00 | 98.18 | 122.82 |
| 3 | 113.94 | 168.94 | 123.91 | 100.66 | 141.96 |
| 4 | 99.32 | 109.80 | 92.15 | 188.66 | 129.63 |
| D | 69.87 | 66.14 | 76.38 | 90.48 | 33.77 |
| Rank | 3 | 4 | 2 | 1 | 5 |
Table 3(b): Shows the response tables for Mean and Signal to Noise Ratios.
| Level | Dextrose | NH4Cl | Metal | Inoculum | Agitation |
| (%) | (%) | ions | (%) | (rpm) | |
| 1 | 41.32 | 38.99 | 43.74 | 40.94 | 39.29 |
| 2 | 43.88 | 39.04 | 41.00 | 38.34 | 41.39 |
| 3 | 39.64 | 44.54 | 41.62 | 38.96 | 40.64 |
| 4 | 38.45 | 40.72 | 36.92 | 45.14 | 41.97 |
| D | 5.43 | 5.55 | 6.82 | 6.80 | 2.68 |
| Rank | 4 | 3 | 1 | 2 | 5 |
 |
Figure 5: Main effect plot for mean (a) and main effect plot for S/N ratio (b) for different factors of Dextrose (A), NH4Cl (B), Metal ions (C), and Inoculum (D), Agitation (E) Numbers 1, 2,3,4 display the levels of each of the medium components A, B, C, D and E used in the study. |
The contribution of five factors in protease production by Taguchi experimental design showed that carbon source played a leading role than the other selected Point prediction of the design showed that maximum protease production of 286.81U/mL was achieved under optimal experimental conditions as compared to before optimization. Hence the selected orthogonal array was L16 and optimum factors for protease production were found to be dextrose, NH4Cl, metal ions, Inoculum% and agitation. The optimum medium condition derived as dextrose (0.6 %), NH4Cl (0.6%), Ca2+ as metal ions, inoculums (8%) and agitation (100 rpm). This result would further facilitate for economic design of process at the large scale level of fermentation.
Acknowledgements
The authors are thankful to Department of Biotechnology, Bapatla Engineering College, Bapatla for providing the infrastructure facilities for this study.
References
- Abdel Fattah Y.R., Saeed H.M., Gohar Y.M. and El Baz M.A., “Improved production of Pseudomonas aeruginosauricase by optimization of process parameters through statistical experimental designs”. Process Biochemistry.40: 1707-1714 (2005).
- Anwar A. and Mohammed S., “Alkaline proteases: a Review”. BioresourceTechnol64: 139-144 (1998).
- Beg Q.K., Sahai V. and Gupta R., “Statistical media optimization and alkalineprotease production from Bacillus mojavensisin a bioreactor”. Process Biochem39: 203–209 (2003).
- Chang M.Y., Tsai G.J. and Houng Y.Y., “Optimization of the medium composition for the submerged culture of Ganodermalucidumby taguchi array design and steepest ascent method”. Enzy Microbial Technol38: 407 414 (2006).
- Cobb B.D. and Clarkson J.M., “A simple procedure for optimizing thepolymerase chain reaction (PCR) using modified Taguchi methods”. Nucleic Acids Res 22: 3801-3805 (1994).
- Dasu V.V., Panda T. and Chidambaram M., “Determination of significantparameters for improved griseofulvin production in a batch bioreactor by Taguchi’s method”. Process Biochem38: 877-880 (2003).
- Erikson N., “Industrial enzymology”. 2nd Ed. London: The Macmillan Press Ltd (1996).
- Frost G.M. and Moss D.A. “Production of enzymes by fermentation. In:Rehm HJ, Reed G, editors. Biotechnology”, Vol. 7a. Weinheim: Verlag Chemie.pp.65– 211 (1987).
- Gupta R., Beg Q.K. and Lorenz P., “Bacterial alkaline proteases: molecular approaches and industrial applications”. ApplMicrobiolBiotechnol59: 15-32 (2002).
- Han J.J., Yang T.H. and Rhee J.S., “Optimization of reaction variables for sucrose monoester production using lipase in a solvent free system by taguchi’s method”. Biotechnol Tech 12(4): 295 299 (1998).
- Koo T.Y., Lin I.P., Liu H.R. and Chou C.Y., “Determination of nattokinase production condition using taguchi parameter design”. Food SciTechnolIntL12(3): 215 220 (2006).
- Jong HyukIm., Jung-Min Song., Jae-Hoon Kang and Dae-Jung Kang., “Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach” .J IndMicrobiolBiotechnol36: 1337-1344 (2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.





