Manuscript accepted on : 02 October 2011
Published online on: --
Antimicrobial Properties of the Leaves of Saraca indica
C. J. Chandekar
Department of Microbiology and Biotechnology, Shri Shivaji Education Society Amravati’s Science College, Congress Nagar, Nagpur - 440 012 India.
Corresponding Author E-mail:chandekarc@yahoo.com.
ABSTRACT: Acetone, chloroform, methanol, petroleum ether and water extracts of leaves of Saraca indica (family-Caesalpiniaceae)were investigated for their antibacterial activity against standard strains of Bacillus cereus, Bacillus subtilis ,Bacillus megaterium, Staphylococcus aureus, Escherichia coli, Proteus vulgaris and Pseudomonas aeruginosa by well diffusion method. Acetone, chloroform, methanol and petroleum ether extracts showed significant antibacterial activity against only Gram positive bacteria.Water extract is found active against Bacillus subtilis and Bacillus megaterium. Fresh leaves juice and acetone extracts showed highest antimicrobial activity against Gram positive microorganisms.Results were compared with standard antibiotics Amoxicillin-Am30, Ciprofloxacin-Cf30, Cotrimaxazole-Co25,Gentamicin-G50 and Tetracycline-T30 . The antibacterial activities of the Acetone, chloroform, methanol and petroleum ether extracts are discussed according to their phytochemical components.It is concluded that the Saraca indica may serve as valuable source of compounds with therapeutic potential.
KEYWORDS: Antibacterial activity; Saraca indica; antibiotics; Acetone; Chloroform
Download this article as:| Copy the following to cite this article: Chandekar C. J. Antimicrobial Properties of the Leaves of Saraca indica. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Chandekar C. J. Antimicrobial Properties of the Leaves of Saraca indica. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9512/ |
Introduction
Herbal medicine has such an extraordinary influence that numerous alternative medicine therapies treat their patients with Herbal remedies, Unani and Ayurveda. Approximately 25 percent of all prescription drugs are derived from trees, shrubs or herbs. Nature has bestowed our country with an enormous wealth of medicinal plants therefore India has often been referred to as the medicinal garden of the world. So stand the medicinal plants Saraca asoca as one of the foremost plants utilized from antiquity till to date (Pradhan,P.et al.2009).
The diverse culture of our country is a rich source of traditional medicine, many of which are of plant origin. Scientific data of such plant derivatives could be of clinical use. Through out the world in current research scenario, the development of a new drug entity with promising efficacy and less toxicity for treatment of various dreadful diseases requires huge investment and longer period of clinical trials. Drugs from natural plants are considered to be a promising source to serve purposes. Among the diversity of most natural products, medicinal plants are still the most favorable for the scientist to explore a new drug. Saraca indica is the one of the most interesting medicinal plant as all part of this plant contain significant & beneficial chemical constituents, which helps in treating various diseases and disorders(Shelar,D.B.et al.2010).
Plant materials have been used for the treatment of serious diseases throughout the world before the advent of modern clinical drugs(Hidayathula S.et al.2011).The use of medicinal plants still plays an important role to cover the basic health needs in the developing countries (Shihabudeen HMS et al.2010).Several top selling drugs of modern times such as Quinine,Artemisinin,Shikonin,etc.are obtained from plants(Hidayathula S.et al.2011). Most of the phytochemicals ,secondary metabolites of plants,are physiologically active(Shihabudeen HMS et al.2010).The plants are known to provide a rich source of botanical,anthelmintic,antibacterials, and insecticides (Acharya S.et al.2011).
Plants are used as medicine since time immemorial. Drugs from plant sources are being used by about 80% of the world population. Herbal medicines have stood the test of time for their safety, efficacy, acceptability and lesser side effects ( Kamboj, V. P.,et al. 2000, Sannomiya, M.,etal.2007).
Saraca indica belongs to the family Caesalpiniaceae.It is found in India, China, Ceylon
and Malaysia. It occurs almost throughout India up-to an altitude of 750 m in the central and in
the eastern Himalayas and Khasi, Garo and Lushai hills, wild in Chittagong, Bihar, Orissa,
Konkan, Deccan, Mysore. It has become quitescarce in several localities and is reported to be
threatened in North Eastern Region of India(Shilpakala Sainath R.et al.2009).
Saraca indica leaves and stem found to contain quercetin, quercetin-3-O-α-Lrhamnoside, kaempferol 3-O- α-Lrhamnoside, amyrin, ceryl alcohol and β-sitosterol (Pradhan, P.et.al.2009, Anonymous,2006, Sadhu, S. K.et al.2007). The antidiabetic, oxytocic, anticancer, peptic ulcer, antimicrobial, antibacterial and antioxidant activities of the plant have been reported (Preethi, F.et al. 2010, Satyavati, G. V.et.al. 1970, Sainath, R. S.et al. 2009, Kaur, J. D.et al.1980, Maruthappan, V.et al.2010, Pal, S. C.et al. 1985, Sandhu, J. K,et al.. 2007)
Materials and Methods
Selection of medicinal plant for this study: Saraca indica
Family
Caesalpiniaceae
Parts used
Leaf
Traditional uses
The plant is useful in dyspepsia, fever, burning sensation, colic, ulcers, menorrhagia, leucorrhoea, pimples etc. The bark,used for pharmaceutical preparations,is bitter, astringent, refrigerant, anthelmintic, styptic, stomachic, constipating, febrifuge and demulcent. Even the juice of the leaves, mixed with cumin seeds, is used for the treatment of stomachalgia (Srivastava G.N.et al.1988).
Chemical constituents
It mainly contains glycosidic principles, non-phenolic, sapogenetic glycoside, sterols and aliphatic alcohols. catechol, (-) epicatechol and leucocyanidin has been isolated from pods and wood contains quercetin. In the powdered bark ash of Ashoka minerals like silica,sodium, potassium, phosphate, magnesium, iron, calcium, strontium and aluminium have been found (N. Indrani, K. et al. 1984, D. J. Kaur, et al. 1980, T. B. Middelkoop,et al. 1985). The flowers contain fatty acids and gallic acid; apigenin-7-O-beta-Dglucoside, cyanidin-3, 5-diglucoside, kaempferol 3-O-beta-D-glucoside, pelargonidin-3, 5-diglucoside, quercetin and its 3-O-beta-D-glucoside and sitosterol. The bark yields alkanes,esters and primary alcohols. It gave n-octacosanol, tannin, catechin, (+)-catechol, (−)-epicatechin, (−)-epicatechol, leucocyanidin, leucopelargonidin, procyanidin derivatives,methyl-and ethylcholesterol derivatives. Quercetin and its 3-O-rhamnoside, kaempferol-3-O-α-L-rhamnoside, amyrin, ceryl alcohol and β-sitosterol have been isolated from leaves and stems (M. Behari, et al.1977).It also contains flavonoids and sterols ( L. Vijai, et al. 1976,M. Behari,et al. 1977).
Identification and Preservation of Plant materials
Fresh plant leaves were collected from the Nagpur area of India. The taxonomic identities of this plant was determined by the expertise of the Post Graduate Department of Botany of Rashtrasant Tukadoji Maharaj Nagpur University,Nagpur. Specimen was labeled, numbered and noted with date of collection, the locally and their medicinal uses and their approximate dosages of administration were recorded. Plant leaves were washed with 70% alcohol and then rinsed with sterilized distilled water, air dried and stored in airtight bottles at 4oC for further use.
Preparation of crude extracts (Fresh juice)
Plant leaves were collected from around Nagpur region in the month of August-September. Leaves were cleaned under running potable water and were washed with 70% alcohol and then rinsed with sterilized distilled water,cut into pieces and grounded in pestle and mortar (made up of dolerite stone) till homogenized mass was obtained. Homogenized mass was squeezed in 400 mesh nylon cloth (pore size 37 micron) to obtain crude extract. Crude extract was kept in sterilized glass bottle. All crude extract were prepared fresh and used before 2 hours. Cold extracts were prepared using individual fresh plant leaves.
Aqueous extraction
Ten grams of dried powder was extracted in 100 ml distilled water for 6 h at slow heat. Every 2 h, it was filtered through 8 layers of muslin cloth and centrifuged at 5000g for 15 min. The supernatant was collected. This process was repeated twice and after 6 h, the supernatant was concentrated to make the final volume one-fourth of the original volume (Shahidi Bonjar GH 2004). It was then autoclaved at 121 ºC and 15 lbs pressure and then stored at 4ºC.
Solvent extraction
Ten grams of dried powder was extracted with 100 ml of each solvent (acetone, chloroform, methanol and petroleum ether)and flasks were kept on a rotary shaker at 190-220 rpm for 24h. Thereafter, it was filtered through 8 layers of muslin cloth and centrifuged at 5000 g for 15 min. The supernatant was collected and the solvent was evaporated to make the final volume one-fourth of the original volume (Shahidi Bonjar, G.H. 2004). It was stored at 4oC in airtight bottles for further studies.
Bacterial cultures
The microbial strains are identified strains and were obtained from the National Chemical Laboratory (NCL),Pune, India. The studied bacterial strains were Bacillus cereus NCIM2155, Bacillus subtilis NCIM2063,Bacillus megaterium NCIM2087, Escherichia coli NCIM2931, Proteus vulgaris NCIM2857 and Pseudomonas aeruginosa NCIM5029. Staphylococcus aureus MTCC96 this strain was procured from Institute of Microbial Technology (IMTECH),Chandigarh,India.They were sub-cultured on nutrient agar for every 15 days and maintained on nutrient agar slants at 40C,fresh inoculums were taken for test.
Media:
Hi -Sensitivity test broth (M 486) and Hi-sensitivity test agar (M 485) were procured from Hi-media Mumbai,India. The media were prepared according to the instructions given.
Screening for the antimicrobial potential of the plant leaves extracts
The antimicrobial activity of different solvent extracts was evaluated by agar well diffusion(Perez C,et.al. 1990& Parekh,J.et al.2007) using Hi-sensitivity test agar (M 485).
Preparation of inoculum
A loopful of culture was inoculated from the stock slant culture in 5 ml of Hi-sensitivity test broth and broth was incubated at 35±0.50C in incubator for 18-20 hrs.After incubation a loopful of actively growing culture was inoculated into 10 ml of Hi-sensitivity broth. Broth was incubated at 35±0.50C for 6-8 hours. This culture was used for the inoculation of Hi-sensitivity test agar plates.
Preparation of Hi-sensitivity test agar medium
Hi-sensitivity test agar medium was prepared as per instructions of manufacturer. Required amount of agar medium was melted and 25 ml of molten medium was distributed in test tubes (25×150 mm). Medium was autoclaved at 15 lb. for 20 min. After autoclaving, medium was maintained at 45-500C in constant temperature water bath.
Inoculation of medium with test organism
0.5 ml of 6-8 hours old test organism is transferred to petridish of 100mm size(Sterilized in oven at 1800C for 1 hr.) using sterile micropipette. Hi-sensitivity test agar medium maintained at 45-500C was poured and mixed properly to ensure uniform distribution of organism with medium. Seeded plates are allowed to set at room temperature.
Preparation of agar well for fresh leaves juice
10 mm borer was used to prepare wells in agar. Four wells per plate at four equidistant corners were made.A 100 µl crude extract(fresh leaves juice) was transferred by micropipette per well. Plates were immediately kept at 40C in refrigerator for 1 hr.for the diffusion of extract and then shifted to 35±0.50C in incubator. Zone of inhibition was measured after 24 hrs. of incubation by zone scale.
Preparation of agar wells for different solvent extracts
5 mm borer was used to prepare wells in agar. Four wells per plate at four equidistant corners were made.
A 50 ul solvent extract was transferred by micropipette per well. Plates were immediately kept at 40C in refrigerator for 1 hr. and then shifted to 350C+0.50C in incubator. Zone of inhibition was measured after 24 hrs. of incubation.For each bacterial strain, controls were maintained in which pure solvents were used instead of the extract.The control zones were sustracted from the test zones and the resulting zone diameter is obtained.
Results
All the microorganisms responded differently to the various plant extracts and standard antibiotics.All the plant extracts and antibiotics tested showed some antimicrobial activity(Table-1).The fresh leaves juice was found to be active against only Gram positive bacteria.When we compared the activity of aqueous extract with fresh leaves juice,the fresh leaves juice is more active.The aqueous extract found to be active against Bacillus subtilis and Bacillus megaterium(Fig-3&Fig-2).
Table 1: Results of antimicrobial activities of fresh leaves juice and solvent extracts of Saraca indica and compared with standard antibiotics.
| Sr.No.Microorganisms Zone of inhibition in mm | |||||||
| Leaves extracts Standard antibiotics | |||||||
| FJ WE AE ME CE PE Am30 Cf30 Co25 G50 T30 | |||||||
| 1.Escherchia coli — — — — — — 32 29 24 17 22 | |||||||
| 2. Proteus vulgaris — — — — — — — 23 31 20 24 | |||||||
| 3.Pseudomonas aerugenosa — — — — — — 14 36 — 34 22 | |||||||
| 4.Staphylococcus aureus 28 — 21 18 14 15 31 23 20 16 17 | |||||||
| 5. Bacillus cereus 25 — 24 15 18 17 15 27 — 23 24 | |||||||
| 6.Bacillus subtilis 28 17 32 28 28 28 31 50 36 40 32 | |||||||
| 7.Bacillus megaterium 23 10 24 21 18 20 29 46 24 23 33 | |||||||
Key: FJ—Fresh juice of leaves; WE—Water extract; AE—Acetone extract;ME—Methanol extract;PE—Petroleumether;Am30–Amoxycilin; Cf30 –Ciprofloxacin; Co25 –Cotrimaxazole; G50 –Gentamicin; Tetracycline–T30 , ND—Not determined; — Negative.
Acetone ,methanol and petroleum ether extracts are active against only Gram positive bacteria Staphylococcus aureus(Fig-4) , Bacillus cereus(Fig-1). Bacillus subtilis(Fig-3) and Bacillus megaterium(Fig-2).All the organisms are susceptible to Ciprofloxacin- Cf30 , Gentamicin-G50 ,and Tetracycline-T30 .Proteus vulgaris is found to be resistant to Amoxycilin Am30 Pseudomonas aerugenosa and Bacillus cereus found to be resistant to Cotrimaxozole Co25.
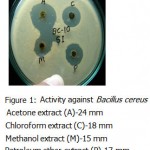 |
Figure 1
|
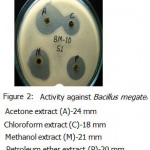 |
Figure 2
|
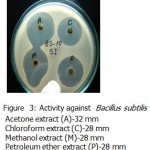 |
Figure 3
|
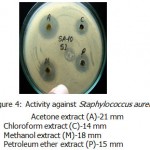 |
Figure 4
|
Discussion
Acetone ,methanol ,chloroform and petroleum ether extracts are active against only Gram positive bacteria Staphylococcus aureus , Bacillus cereus,. Bacillus subtilis and Bacillus megaterium.All the Gram negative microorganisms ie. Escherchia coli Proteus vulgaris and Pseudomonas aerugenosa are found resistant to fresh juice of leaves and acetone ,methanol ,chloroform and petroleum ether extracts.Seetharaman et al.and Sarojini Nayak et al.found that the ethanolic extract of leaves was active against Escherchia coli and Staphylococcus aureus. Pal et al.and Sarojini Nayak et al. saw that the methanolic extract was active against Bacillus subtilis.Moreover, Sarojini Nayak et al. found that the methanolic extract was more effective in cases of Staphylococcus aureus and Escherichia coli. Rajesh Dabur et al.found that the petroleum ether extract is active against Staphylococcus aureus and Proteus vulgaris.Methanol extract is found active against Pseudomonas aerugenosa, Staphylococcus aureus and Proteus vulgaris. Chloroform extract is found inactive against Pseudomonas aerugenosa, Staphylococcus aureus and Proteus vulgaris.They also found that water extract is more active against Escherchia coli Proteus vulgaris, Pseudomonas aerugenosa, Staphylococcus aureus and Bacillus cereus.
The fact that the aqueous extract is more active than methanol extract implies that the antimicrobial action may be due to the synergistic action of different chemical constituents, some of which probably are lost upon extraction with solvent (Bibitha B et al.2002).
Different solvents have been reported to have the capacity to extract different phytoconstituents depending on their solubility or polarity in the solvent(Marjorie,M.C.1999). Acetone extracts in this study might have had higher solubility for more phytoconstituents, consequently the highest antibacterial activity. The demonstration of antimicrobial activity by water extracts provides the scientific basis for the use of these plants in the traditional treatment of diseases, since most traditional medicine men use water as their solvent in which the decoctions are prepared.In our study fresh leaves juice and acetone extracts showed highest antimicrobial activity against Gram positive microorganisms.
Plants are used medicinally in different countries and are a source of many potent and powerful drugs (Srivastava,J.et al.1996, Reuben, K.D.,2008 ). Natural products either extract or pure compounds provide unlimited opportunities for the development of new drugs due to the availability of chemical diversity(Cos, P., et al.2006). To overcome the problem of antibiotic resistance ethnic medicinal plants have been extensively studied as an alternative treatment for diseases due to their ability to produce a variety of compounds of known therapeutic properties
( Harbone, S.B.et al.1995& Kumar, B.,et al.2007) and much attention has been paid to plant extracts and their biologically active compounds(Suresh,G.et al.2010). The screening of natural products has been the source of innumerable therapeutic agents(Korosecchviz,J.I.et al.1992) . Higher plants as a source for new potential drugs is still largely unexplored and only a small percentage of them has been subjected to phytochemical investigation and the fractions submitted to pharmacological screening is very low. Such screening of various natural organic compounds and identifying active agents is a need of the hour as due to successful prediction of lead molecule and drug like properties at the onset of drug discovery will pay of later in drug development.
Acknowledgement
I am thankful to University Grants Commission, New Delhi, India for financial assistance rendered to us under the scheme of minor research project.
References
- Acharya S,Dash G.K.; Brahma D.K.;Chhetree R.R.Preliminary phytochemical investigation and anthelmintic activity of Acacia suma (Roxb)barks .IRJP 2011;2(1):136-141.
- Anonymous,Wealth of India ,A Dictionary of Indian Raw Materials and Industrial Products,National Institute of Science Communication and information Resources, CSIR, New Delhi 2006.
- Behari,M; C. K. Andhiwal, J. A. Ballantine. J. Chem., 1977, 15B, 765
- Bibitha B, Jisha VK, Salitha CV, et al. Antibacterial activity of different plant extracts. Indian J Microbiol 42(4) 361-363,2002..
- Cos, P., Vilietinck, A J., Vanden Berghe,D., and Maes, L., 2006. Anti-infective potential of natural products: how to develop a stronger in vitro proof-of concept. Journal of Ethanopharmacology,106: 290-302.
- Dabur R., Gupta,A.; Mandal, T K; Singh* D. D.Singh, Bajpai#,V.; Gurav,A.M. Lavekar,G.S,;Traditional,ComplementaryandAlternativeMedicines.2007;4(3):313-318.
- Harbone, S.B. and Baxer, H., 1995.Phytochemical dictionary. A handbook of bioactive compounds from plants.Taylor and Francis, London.
- Hidayathula S.;Chandra K.K.;Chandrashekar K.R.;Phytochemical evaluation and antimicrobial activity of Pterospermum diversi folium blume.Int J Pharma Pharma Sci 2011;3(2):165-167.
- Indrani N, Balasubramanian, Leather Sci., 1984, 31, 349.
- Kamboj, V. P., Current Science 2000, 78, 35-39.
- Kaur, J. D., Misra, K., Indian Chem. Soc.1980, 57, 1243.
- C.P.; Indian Medicinal Plants. An Illustrated Dictionary. Springer Science,Springer-Verlag Berlin/Heidelberg, 2007.
- Korosecchviz, J. I., and Howe-Grant, M.,1992. Kirk-othmer Encyclopedia of Chemical Technology, 2: 893 632.
- Kumar,B.,Vijaykumar,M.,Govindarajan,R.andPushpangadan,P.,2007.Ethnopharmacological approaches to wound healing-Exploring medicinal plants of India. Journal of Ethnopharmacology, 114, (2):103-113.
- Marjorie M .C. Plant products as antimicrobial agents. Clin Microbiol Rev, 12(4): 564-582
- Maruthappan, V., Shree, K. S., J. Pharm. Res.2010, 3, 17-20.
- Middelkoop,T.B.; R. P. Labadie, Z. Naturforsch B: Anorg Chem, Org. Chem., 1985, 40B, 855.
- Nayak,S.;Sahoo,A.M.,J.Pharm.Res.and Dev.2011;vol3(3),160-163.
- Pal, S. C, Maiti, A. P., Chatterjee, B. P., Nandy,A., Indian J. Med. Res. 1985, 82, 188-189.
- Parekh J, Chanda S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk J Biol 31: 53-58, 2007.
- Perez C, Paul M, Bazerque P. Antibiotic assay by agar well diffusion method. Acta Bio Med Exp 15: 113-115, 1990.
- Pradhan,P.;L.Joseph;V.Gupta;R.Chulet;H.Arya;R.Verma;and A.Bajpai;Saraca asoca(Ashoka:A Review.J.Chem.and Pharm. Res.2009,1(1) :62-71.
- Preethi, F., Fernandes, J., Pricilla, K., Pharm.Res. 2010, 3, 491-493.
- Reuben, K.D., Abdulrahman, F.I., Akan,J.C., Usman, H., Sodipo, O.A. and Egwu,G.O.,2008. Phytochemical screening and in vitro antimicrobial investigation of the methanolic extract of Croton zambesicus Muell. ARG. stem bark. Eur. J. Sci. Res.,23: 134-140.
- Sannomiya, M., Cardoso, C. R. P., Figueiredo,M. E., Rodrigues, C. M., dos Santos, L. C., dos Santos, F. V., Serpeloni, J. M., Colus, I. M. S.,Vilegas, W., Varanda, E. A., Ethnopharmacol. 2007, 112, 319-326.
- Satyavati, G. V., Prasad, D. N., Sen, S. P.,Indian J. Med. Res. 1970, 58, 660-663.
- Sainath, R. S., Prathiba, J., Malathi, R., Rev. Med. Pharmacol. Sci. 2009, 13, 371- 374.
- Sadhu, S. K., Khatun, A., Phattanawasin, P.,Ohtsuki, T., Ishibash, M., Nat. Med. 2007, 61,480-482.
- Seetharam N, Sujeetha H, Jyothiswaran G, Barad A, Sharanbasappa G, Praveen S, Antibacterialactivity of Saraca Asoka Bark, Ind.J.Pharm.Sci.,65(6), 2003, 658-659.
- Shahidi Bonjar GH. Evaluation of antimicrobial properties of Iranian medicinal plants against Micrococcus luteus, Serratia marcescens, Klebsiella pneumoniae and Bordetell bronchiseptica.Asian J Plant Sci 3: 82-86, 2004.
- Shihabudeen HMS,Priscilla D H,Thirumurugan K.Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants.Int J Pharma Sci Res 2010;1(10):430-434.
- Shilpakala S.R;Pratibha J.;Malathi R.Antimicrobial properties of the stem bark of Saraca indica(Caesalpiniaceae).Europen review for Medical and Pharmacological Sciences.2009,13:371-374.
- Shelar,D.B; P.J.Shirote;N.S.Naikwade.Anti-inflammatory activity and brine shrimps lethality test of Saraca Indica Linn leaves extract.J.Pharm.Res.2010,3(8),2004-2006.
- Srivastava, J., Lambert, J. and Vietmeyer,N., 1996. Medicinal plants: An expanding role in development. World Bank Technical Paper, No. 320.
- Srivastava G.N.Bagchi G.D. Srivastava A.K.Pharmacognocy of Ashoka stem bark and its adulterants. Int J Crude Drug Res 1988;26(2):65.
- Suresh, G., Ramesh, B., Kavitha, K.,Ravichandran, N., R., Suresh, A.,Gopalakrishnaan, V. and Vijaiyan Siva,G., 2010. Preliminary screening of antibacterial compounds from Palar river basin flora. Journal of Phytology,2(2): 24-29.
- Vijai,L.; J. S. Chauhan, Ind. Chem. Soc., 1976, 53,

This work is licensed under a Creative Commons Attribution 4.0 International License.





