Manuscript accepted on : April 22, 2011
Published online on: 28-06-2011
F. O. Omoya
Department of Microbiology, School of Sciences, Federal University of Technology, P. M. B. 704, Akure, Ondo State Nigeria.
ABSTRACT: One of the greatest challenges facing Africa in the fight against malaria is drug resistance. Resistance to chloroquine, the cheapest and most widely used antimalarial, is common throughout Africa. As a result of this trend, other control strategies are being considered. Biological control of malaria vector, an integral part of controlling malaria has rarely been exploited in Nigeria. Recent developments in this field show that certain fungi displayed activities against Anopheles mosquitoes. Investigation of bioactivities of two entomopathogenic fungi namely Varicosporium elodeae and Articulospora inflata were assessed in vitro on pupal stage of Anopheles mosquitoes in this research. The fungi were applied separately to the pupae. The treatments were conducted in a controlled environment for five days during which mortality of the pupae was checked. The result revealed that the two treatments were significantly different from each other. High mortality was recorded in the groups treated with V. elodeae within 24 hours of post-treatment. In contrast, death of the pupae started after 48 hours of treatment with A. inflata. Varicosporium elodeae appeared more potent than A. inflata; the lethal dose concentrations obtained for the former and latter fungi were LC50 (2.64 sfu/ml) and LC50 (5.486 sfu/ml) respectively. Conclusively, V. elodeae and A. inflata biopesticide can potentially be an important component of malaria control strategy in Nigeria.
KEYWORDS: Entomopathogenic fungi; malaria; drug resistance and Bioactivity
Download this article as:| Copy the following to cite this article: Omoya F. O. Susceptibility of Anopheles Arabiensis Mosquito Pupal Stage to Bioactivities of Varicosporium Elodeae and Articulospora Inflata. Biosci Biotech Res Asia 2011;8(1) |
| Copy the following to cite this URL: Omoya F. O. Susceptibility of Anopheles Arabiensis Mosquito Pupal Stage to Bioactivities of Varicosporium Elodeae and Articulospora Inflata. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=9277 |
Introduction
A recent upsurge of malaria in endemic disease area with explosive epidermics in many parts of Africa is probably caused by many factors, including rapidly spreading resistance to antimalaria drugs. Malaria in human is caused by a protozoan of the genus Plasmodium and the four subspecies, falciparum. vivax, ovale and malariae. Plasmodium falciparum is responsible for the greatest illness and death in Africa. The disease is transmitted by the bite of female Anopheles mosquito of which Anophelesgambiae complex is the most efficient in the transmission of this disease in Africa (WHO, 2006). Malaria is an important social, economic and developmental problem affecting individuals, families, communities and countries. In the last decade, the prevalence of this disease has been escalating at an alarming rate, especially in Africa. An estimated 300 to 500 million cases each year cause 1.5 to 2.7 million deaths, more than 90% in children under 5 years of age in Afica (WHO, 2006); it is ranked third among major infectious disease threats in Africa after pneumococcal acute respiratory infections (3.5%) and tuberculosis (TB) (2.8%). Cases in Africa account for approximately 90% of malaria cases in the world (Thomas, 1998). There is a wide variation in the clinical manifestation of the disease, ranging from asymptomatic malaria, uncomplicated and with the most severe forms characterized by life-threatening complications; severe malaria anemia and cerebral malaria (Amoduet al., 2005).
The control of malaria has been a preoccupation of man; various efforts have been put in place to reduce the scourge of this infection especially among pregnant women and children less than 5 years (Idowuet al., 2008). In the past, emphasis was placed on the eradication of malaria; presently the main thrust is to control the disease. However, these strategies have not yielded sufficient and encouraging results considering the morbidity – mortality ratio. Vector control therefore remains one of the main elements of the malaria control strategy, particularly in areas of unstable and less intense transmission (Idowuet al., 2008). According to WHO (2006), vector control remains the most generally effective measure to prevent malaria transmission and is therefore one of the four basic technical elements of the Global Malaria Control Strategy GMCS. Vector control represents an important part of the current global strategy for the control of major vector-borne diseases, and has a vital role in the prevention of malaria (WHO, 2006). The current study is to investigate the use of two entomopathogenic fungi Varicosporiumelodeea and Articulosporainflataas a control method against malaria vector in Nigeria, by infecting the pupal stage of Anopheles mosquito with their conidia.
Material and Methods
The isolation of entomopathogenic microorganisms was conducted in 2007 at the Federal University of Technology, Akure (FUTA), Nigeria. Cockroaches and housefly were collected into sterile containers from their natural breeding habitats (cupboards for cockroach and housefly around the refuse dumps) in FUTA. In the laboratory, adult cockroaches were placed inside a sterile petri dish containing 10mL of sterile water each (in triplicate). The petri dish was properly shaken to ensure good washing away of particles that were on the cockroaches. One millimetre was taken from the wash water, serially diluted to 10-4 and 0.1ml of the 10-4 serial dilution was pour plated using potato dextrose agar. Incubation was done at 25oC for 72 h (fungi), after which the agar was observed for growth. Identification of the fungi was done by comparison of the observed morphological characteristics with those described by Onions et al. (1995), after examination under the microscope. The same procedure was repeated using housefly.
Fungal cultures were grown on Potato dextrose agar (200 g of potato, 20 g of glucose, 20 g of agar and 1000ml of distilled water) at 25oC. Conidial suspension was obtained by scraping conidia from 12 days old well sporulated cultures into an aqueous solution of 0.2 % Tween-80. The suspension was then filtered through muslin cloth to remove mycelium and the concentrations of viable conidia was estimated as spore forming units, using a dilution plate count method.
The mosquito larvae were collected from stagnant waters. They were selected and differentiated using both physical and molecular characterisations. The Anopheles arabiensispupae were reared in a meshed cage at 25oC and 70% relative humidity under 14L : 10D photoperiod with slight modifications according to Zhonget al. ( 2006). They were fed daily with Tetramin® fish food. This allowed them to reach maturity stage after which they were offered blood meal. Eggs laid on wet filter papers were transferred to water trays. Pupae were manually into containers containing sterile water.
The pupae were surface sterilised in separate petri dishes using 75% alcohol and rinsed with sterile water. There were three replicates and the control per treatment with 25 pupae in a container making up a replicate. One hundred pupae constituted a group. Susceptibility of the mosquitopupaeto thefungal isolates was evaluated by subjecting the 25 pupae to fungi. Each group was inoculated with each of the varying conidia loads of 1.3 to 6.5 Sfu/ml. Incubation was carried out for 5 days at 27oC. Dead cadavers were removed daily and counted. The LC25, LC50 and LC75 of the fungi were determined using probit analysis (Finney, 1971).
Results and Discussion
Application of the two pathogens, V. elodeae and A.inflata caused considerable mortality effect on the pupal stage of the Anopheles mosquito. Spore population and incubation time affected the percentage mortality of the pupae (Fig. 1-2). There was increased mortality as the spore load increased. The digestion of mosquito was rapid when sufficient high spore number was used. Varicosporiumelodeaeproduced higher number of dead pupa even within 1 day of post-infection than A.inflata in all the treated groups. This is supported by the lethal dose (LC50)of 2.64 Sfu/ml for V. elodeae and 5.486 sfu/ml for A.inflata (Table 1). This means that V. elodeae was more effective and its spores infected their pupae better than A.inflata.
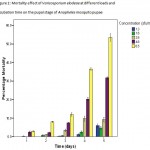 |
Figure 1: Mortality effect of Varicosporiumelodeae at different loads and incubation time on the pupal stage of Anopheles mosquito pupae.
|
 |
Figure 2: Mortality effect of Articulosporainflataat different loads and incubation time on the pupal stage of Anopheles mosquito pupae.
|
Table 1: Relative potency of Varicosporiumelodeae and Articulosporainflate on the pupal stage of Anopheles mosquito larvae.
| No Line name | LC25 Lower limit Upper limit 1 2 Index | RR Slope LC50 LC75 | |
| 1 Varicosporiu m elodeae | 0.273
0.56 * |
0.043
* 100 |
1 1.123 2.64 3.43 |
| 2 Articulosporainflata
0.926 |
0.542
0.837 5.486 |
0.095
* 52.127 14.654 |
2.865
|
| Index compared with Varicosporiumelodeae Resistance Ratio (RR) compared with Varicosporiumelodeae | |||
Higher mortality was noticed with increase in contact time of the fungi (Fig. 1 and 2). The percentage mortality was highest in treatments with V.elodeaewith values significantly different from A.inflata at p£0.05. This disparity in activity displayed by V. elodeaeand A.inflataon the pupae at higher concentrations may be due to the fact that V. elodeae was able to digest the hard cuticle better the other fungus. Similar observation was reported by Manonmaniet al. (2008). The result of the present work demonstrated that Anopheles arabiensis pupae are susceptible to V. elodeaeand A.inflata, incubation time and fungal concentrations affected the level of mortality.
References
- WHO (World Health Organisation), Malaria vector control and personal protection: Report of a World Health Organization study group. WHO Technical Report Series, 936 (2006).
- Amodu, O. K., Adeyemo, A. A, Gbadegesin, R. A., Orimadegun, A. E., Akinsola, A. K., Olumese, P. E. and Omotade, O. O., Genetic diversity of the msp-1 locus and symptomatic malaria in south-west Nigeria. ActaTropica,95: 226-232 (2005).
- Finney, D. J., Probit analysis. 1stEdition. Cambridge University Press, Cambridge, London. 100-121 (1971) .
- Idowu, O. A., Mafiana, C. F., Luwoye, I. J. and Adehanloye, O., Perceptions and home management practices of malaria in some rural communities in Abeokuta, Nigeria. Travel Medicine and Infectious Disease, (2008). In-press.
- Onions, A. H., Allsopp, D and Eggins, H. O. W., Smith’s Introduction to Industrial Mycology. 2nd edition, The Pitman Press, Bath.65-92 (1995).
- Thomas, C. N., Malaria: a reemerging disease in Africa. World Health Organisation, 4(3): 187-192 (1998).
- Zhong, D., Temu, E. M., Guda, T., Gouagna, L., Menge, D., Pai, A., Githure, J., Beier, J. C. and Yan, G., Dynamics of gene introgression in the African malaria vector Anophelesgambiae. Genetics, 172: 2359-2365 (2006).
- Manonmani, A. M., Prabakaran, G. and Hoti, S. L., Retention of mosquito larvicidal activity of lyophilized cells and WDP formulation of Bacillus thuringiensisvar. israelensison long-term storage. ActaTropica, 105: 170-175 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.





