Manuscript accepted on : August 12, 2010
Published online on: 28-12-2010
Production of Laccase from Newly Isolated Fungal Strain Aspergillus Foetidus - 3397
Nand Lal1* and K. D. S. Yadav2
1Department of Chemistry, V.S.S.D.College, Kanpur India.
2Department of Chemistry, D.D.U. Gorakhpur University, Gorakhpur India.
ABSTRACT: Laccase is an oxidase enzyme, produced from newly isolated fungal strain Aspergillus foetidus. Maximum activity of laccase appeared on the 9th day of inoculation of spores of fungal strain in the liquid culture medium. The activity of laccase detected by UV/VIS spectrophotometer using guiacol as a substrate. The enzymatic characteristic like Km, pH and temperature optimum were reported for this enzyme.
KEYWORDS: Aspergillus foetidus; Oxidative enzyme; laccase; enzyme unit; temperature optima; pH optima
Download this article as:| Copy the following to cite this article: Lal N, Yadav K. D. S. Production of Laccase from Newly Isolated Fungal Strain Aspergillus Foetidus - 3397. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Lal N, Yadav K. D. S. Production of Laccase from Newly Isolated Fungal Strain Aspergillus Foetidus - 3397. Biosci Biotech Res Asia 2010;7(2). Biosci Biotechnol Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9573 |
Introduction
Laccase is a copper-containing enzyme 1,4-benezenediol oxidase (EC 1.10.3.2), found in many plants, fungi and microorganism1-4. It is catalyses the oxidation of phenolic compounds such as ortho and para-diphenols to their corresponding quinones with the concomitant reduction of oxygen to water1. Phenoloxidase3,4 and peroxidase (lignin and manganese peroxidase) are, two oxidative enzymes (laccases) have the greatest attention. Laccases are involved in the biotransformation of many persistent environmental pollutants, like azodyes8, polycyclic aromatic hydrocarbons9,10 and in the biodegradation of lignin4,11 wood and lignocellulosics compounds12,13. Laccases can be used in bioremediation14.
It is proposed that laccases play a role in the formation of lignin by promoting the oxidative coupling of lignols, a family of naturally occurring phenols15. Laccases have been examined as the cathode in enzymatic biofuel cells. They can be paired with an electron mediator to facilitate electron transfer to a solid electrode wire16. This enzyme can be used for textile dyeing/ textile finishing, wine cork making, teeth whitening and many other industrial, environmental diagnostic and synthetic uses.17Thus there is a need to isolate some special types of fungal strain which can produce laccases.
Material and Methods
Yeast extract powder type was from Himedia Laboratory Pvt.Ltd. Mumbai, peptone (beacteriological) was from Qualigens, Mumbai, agar-agar (Bacteriological grade) was from India Drugs and Pharmaceuticals Ltd. Hyderabad. All other chemical used are either from CDH (Delhi) or LobaChemie (Mumbai).
Isolation of Fungal Strain
The soil sample which are used for isolation of fungal strain was collected from the waste disposal sites of paper and chemical industries. 2g of sawdust was taken in 100 ml of a conical flask and 10 ml of double distilled water was added to it. The culture flask was sterilized and 1 g of soil sample was spread over the surface of the sterilized sawdust. The operation was done aseptically inside a laminar flow (Microfilt India, Pune vertical model (2’x2’x2’)) and the culture flask was incubated in a BOD at 300C. A parallel control experiment was also set up in which no soil sample was spread over the sterilized sawdust kept in another culture flask. After 5 days different fungal colonies were observed in the flask containing the soil sample. These fungal colonies were purified by transferring them repeatedly onto agar plates. The agar composition was as reported by Tien and Kirk18 and consisting of the following components: glucose 10g, malt extract 10g, peptone 2g, yeast extract 2g, asparagines 1g, KH2PO4 2g, MgSO4. 7H2O 1g, thimine-HCl 1 mg and agar 20g per liter and were maintained on agar slopes as reported by Tien and Kirk. Four pure fungal strains were purified, out of which one fungal strain was found to secrete laccase in the YED19 liquid culture medium. The fungal strain was got identified as Aspergillusfoetidus by the MTCC centre at the Institute of Microbial Technology, Chandigarh (India) and is deposited there with a number MTCC-3397.
Laccase Activity Determination
YED liquid culture medium which consisted of 0.5g glucose, 0.5g yeast extract and 1g of sawdust in 50 ml double distilled water in 250 ml culture flasks were sterilized and 1 ml of spore suspension (6.7 x107 spores/ml) was added to the each culture flasks aseptically. The spore suspension was prepared by adding 2ml of 1% twin 80 solution in sterilized double distilled water on agar slope containing the spores of the fungi. The spores were counted by putting the spore suspension in haemocytometer and observing under light microscope. The spore density was calculated by finding the average counts of the spores. The culture flasks were incubated in a BOD at 300C and were allowed to grow under stationary growth culture conditions (three culture flasks were set). 2ml of aliquots of the culture medium were withdrawn at regular time intervals and were filtered through sterilized millipore filter (0.22 µm). The filtered extract was analysed for the activity of laccase using UV/VIS spectrophotometer Hitachi (Japan) Model U-2000. The enzyme assay solution 1 ml contained 10 mMguiacol in 40 mM sodium phosphate buffer pH 2.0 maintained at 250C. The enzymatic reaction was initiated by adding 0.2ml of the culture filtrate. The reaction was monitored by measuring absorbance changes at 470 nm with time. The enzymatic activity was calculated using molar extinction coefficient value of 2.66 x 104 M-1 cm-1. One enzyme unit is the amount of enzyme that produces 1µ mole of the product per minute. Each assay was done in triplicate.
Preparation of Laccase and Studies of Its Enzymatic Characteristics
A.foetiduslaccase used in these studies was prepared by growing the fungus following the method described as in above section. On the 9th day of inoculation of spores when the maximum activity of laccase appeared in the liquid culture medium, the culture flasks were taken out the BOD and mycelia were removed by filtration through single use sterile millipore membrane filter unit (0.22µm). The enzyme extract was kept in a fridge at 40C and remained fully active for a month.
The enzymatic characteristic like Km, pH and temperature optimum were determined using guiacol as the substrate for assessing the experimental conditions suitable for biotechnological applications of this enzyme. The enzyme assay solution and assay methods were same as mentioned above. The steady state velocity of the enzyme catalyzed reaction was determined at different substrate concentrations. The Km value was determined from the double reciprocal plot following the standard procedure20. For determine the pH optimum of the enzyme steady state velocity of the enzyme catalyzed reaction was measured at a fixed saturating concentration of the substrate at a fixed temperature and varying the pH of the reaction medium by using suitable buffers. Similarly temperature optimum was determined by measuring the steady state velocity of the enzyme catalyzed reaction using a fixed saturating concentration of the substrate at a fixed pH and varying the temperature of the reaction medium.
Results and Discussion
Laccaseactivity were detected in the filtered enzyme extract when A.foetidus strain were grown in the liquid culture medium. From fig(1) it is obvious that maximum activity appears on the 9th day after inoculation of the fungal mycelia. The peak value of the laccase activity is 17 mU/ml, indicating that Aspergillusfoetidus strain is a source of production of extracellular laccase. In order to finding out the conditions under which the enzyme will exhibit its maximum activity, pH and temperature optimum of the enzyme were determined. Fig.(2) shows the variation of enzyme activity with pH, which indicate that the pH optimum for the enzyme is about 2.2, and it has appreciable activity in the pH range 1.0 to 3.0. Thus laccase enzyme of reported fungal strain is suitable for biotechnological applications in the acidic range.
 |
Figure 1: Production of Laccase by Aspergillusfoetidus.
|
 |
Figure 2: Activity – pH profile of Aspergillusfoetidus.
|
The variation of activity of laccase produced by A. foetidus with temp is shown in fig(3), indicates that the temperature optimum of this laccase is 650C and it has reasonable activity in the temperature range 250C to 750C. Thus this enzyme can used in the higher temperature range.
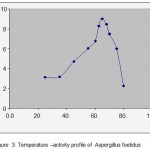 |
Figure 3: Temperature –activity profile of Aspergillusfoetidus.
|
Fig (4) shows the Michaclis-Mentenbehaviour of the laccase produced by A. foetidus using guaicol as the substate. The double reciprocal plot given in the fig(4) shows that the laccase obey Michaelis-Menten equation and has Km value of 0.08 mM. Lower value of Km, shows that A. foetiduslaccase has greater substrate affinities.
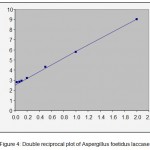 |
Figure 4: Double reciprocal plot of Aspergillusfoetiduslaccase.
|
Conclusion
Newly isolated fungal strain has ability to produce laccases enzyme which are active in acidic range. Temperature optima of this enzyme is very high. This laccase enzyme can apply up to higher temperature range. Substrate affinities is very high of laccase enzyme produced by newly isolated fungal strain.
Acknowledgements
The authors are thankful to the department of Environment and forest, Govt. of India, New Delhi for the financial Support.
References
- ZofiaOlempska-Beer. Chemical and Technical Assessment, 61stJECFA.FAO (2004).
- Mariana Mansur et.al.Mycologia95(6): 1013-1020 (2003).
- Thurston CF. Microbiology. 140: 19-26 (1994).
- Leonowicz A., et.al. J.BasicMicrobiol. 41: 185-217 (2009).
- Reddy CA. J.Biotechnol. 30: 91-107 (1993).
- Reddy CA, D’Souza T.M. FEMS Microbiol Rev. 13: 137-152 (1994).
- Cullen D. J.Biotechnol. 53: 273-289 (1997).
- Michael M. et.al. Appl. & Environ. Microbiol.71(5): 2600-2607 (2005).
- Alcadle M. et.al. J.Biomol Screen, 7(6): 547-553 (2002).
- Hunsa, P. et.al. African Journal Biotechnology.8(21): 5897-5900 (2009).
- Bourbonnais R, Paice M.G. FEBS Lett267: 99-102 (1990).
- Delgado G, et.al. Kuwahara M, Shimadam Eds. Biotechnology in Pulp and Paper Industry. Tokyo: UNI Publishers. 209-214 (1992).
- Klibansky M. et al. Acta Biotechnological.13: 69-76 (1993).
- Suresh PS. et.al. J.Mol.Graph. Model. 26(5): 845-9 (2008).
- Edwards I, et al. Chemical Reviews. 96:
2563-2606 (1996). - Wheeldon, I.R. et.al. Proceedings of the National Academy of Science of the USA.105(40): 15275-15280 (2008).
- Xu,Feng. Industrial Biotechnology (Mary Ann Liebert, Inc).1(1):38-50 (2005).
- Tien, M. and Kirk, T.K. Methods in Enzymol.161: 2239 (1988).
- Pedro M.C. et. al. Microbial. 59(8): 2607 (1993)
- Engel P.C. Enzyme Kinetics, Chapaman& Hall, London. (1979).

This work is licensed under a Creative Commons Attribution 4.0 International License.





