Manuscript accepted on : December 04, 2010
Published online on: 28-12-2010
Phytochemical Investigation of Methanolic Extract of the Leaves of Evolvulus Alsinoides Linn.
N. Vijayalakshmi1 and J. M. Sasikumar2
1Research and Development Centre, Bharathiar University, Coimbatore - 641 046 India. 2Department of Biotechnology, School of Life Sciences, Karpagam University, Coimbatore - 641 021 India. Corresponding Author E-mail: jmsashikumar@yahoo.co.in
ABSTRACT: The present study deals with the phytochemical investigation and therapeutic importance of Evolvulus alsinoides L., an important medicinal plant. Worldwide trend towards the utilization of natural plant remedies has created an enormous need for the use of medicinal plants. This study involves the preliminary phytochemical screening, separation and identification of compounds present in the crude extract of E.alsinoides leaves by HPTLC. Qualitative analysis of the methanolic extract prepared from E.alsinoides leaves revealed the presence of alkaloids, flavonoids, tannins, steroids, glycosides, terpenoids, saponins, reducing sugars, amino acids, gums and mucilages. The results obtained after qualitative analysis was confirmed by spectral analysis. The presence of multiple peaks of polyphenolic compounds was observed in HPTLC fingerprinting. The results suggested that the phytochemical properties of the leaves are responsible for curing various ailments. The plant E.alsinoides is a potential source of various types of bioactive compounds with diverse chemical structures as well as pharmacological activities.
KEYWORDS: Evolvulus alsinoides L; HPTLC; polyphenols; phytochemical analysis
Download this article as:| Copy the following to cite this article: Vijayalakshmi N, Sasikumar J. M. Phytochemical Investigation of Methanolic Extract of the Leaves of Evolvulus Alsinoides Linn. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Vijayalakshmi N, Sasikumar J. M. Phytochemical Investigation of Methanolic Extract of the Leaves of Evolvulus Alsinoides Linn. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9379 |
Introduction
World plant biodiversity is the largest source of herbal medicine and still about 60 – 80 % world population rely on plant based medicines which are being used since the ancient ages as traditional health care system [1]. Indian plants have always provided a base for synthesis of biologically active drugs. The medicinal value of these plants lies in the bioactive phytochemical constituents that produce definite physiological effects on human body. These natural compounds formed the base of modern drugs [2]. Since there is increase in the use of synthetic drugs leading to undesirable hazards, there is a worldwide trend to go back to natural resources mainly traditional plants, which are both culturally acceptable and economically viable.
Plants synthesize primary metabolites which are transformed into secondary metabolites that are used as drugs. The most important of these include: alkaloids, glycosides, saponins, phenolics, terpenoids, lignins, steroids, flavanoids, tannins, gums and mucilages [3]. These are the phytochemicals which are biologically active non-nutrients and antioxidant constituents in plant that have raised interest among Scientists, food manufacturers, producers and consumers for their role in protection and maintenance of human health. The phytochemical research based on ethno-pharmacological information is generally considered an effective approach in the discovery of new anti-infective agents from the plants [4]. High performance thin layer chromatography [HPTLC] method has emerged as an important tool for the qualitative and quantitative phytochemical analysis of herbal drugs and formulations. It is an invaluable quality assessment tool for the evaluation of botanical materials. The major advantage of HPTLC is that several samples can be analysed simultaneously using a small quantity of mobile phase. It is accurate, precise, sensitive, simple, easy to perform, rapid, versatile and cost effective.
Evolvulus alsinoides L. (family : Convolvulaceae) is a Dwarf morning glory, a perennial herb with small woody and branched rootstock, grows widely in open grassy places throughout India [5]. It is used as a brain tonic in the treatment of neurodegenerative diseases [6]. The leaves are used in treating chronic bronchitis and asthma. The root is used for childhood fever, cough, cold and the oil stimulates the growth of hair [7]. Using the whole plant in the form of a decoction with cumin and milk, is used to treat nervous debility, loss of memory and syphilis. It is used medically for curing azoospermia, adenitis, depression and venereal diseases [8]. It is helpful in general weakness, urinary disorders and hypertension. Pre-clinical investigations of E.alsinoides have demonstrated anti-amnesic [9], antistress, anthelmintic [10], antiulcer, anticatatonic [11], antioxidant [12], immunomodulator [13], antibacterial [14] and gastro protective activities [15]. Very less research has been done on this plant leaf and due to leaves property in curing different ailments, this part was selected for the study. The present investigation has been carried out to shed light on the preliminary phytochemical analysis of E. alsinoides leaves.
Materials and Methods
Collection of plant samples
The plant Evolvulus alsinoides L. was collected from Coimbatore District, Tamil Nadu in the month of September 2008. It was identified by the Botanical Survey of India, Coimbatore and a voucher specimen (No.BSI/SC/5/23/09-10/Tech.133) was kept for future reference.
Plant extraction
The leaves were shade dried at room temperature and coarsely powdered. Ten gram of dried powdered sample was macerated with 50 ml of methanol for 24 hrs at room temperature. The extract was centrifuged (3000xg) thrice and the clear supernatants were combined. The combined supernatants were filtered through Whatman No.1 filter paper and the residue was re-extracted with the same solvent. All extracts were combined together and left to dry. The resultant crude methanol extract was used for phytochemical analysis.
Preliminary phytochemical screening
Pytochemical tests was carried out in the methanolic extract of the powdered form of leaf sample using standard qualitative methods [16, 17].
Qualitative analysis of phytochemical constituents
Test for flavonoids
1 ml of the extract was added with few drops of dilute sodium hydroxide. An intense yellow colour which become colourless on addition of a few drops of dilute hydrochloric acid indicates the presence of flavonoids.
Test for alkaloids
5 ml of the extract was added to 2 ml of Hydrochloric acid. To this acidic medium, 1 ml of Dragendroff’s reagent was added. An orange or red precipitate produced immediately indicates the presence of alkaloids.
Test for steroids
1 ml of the extract was dissolved in 10 ml of chloroform and equal volume of concentrated sulphuric acid was added by sides of the test tube. The upper layer turns red and sulphuric acid layer showed yellow with green fluorescence. This indicates the presence of steroids.
Test for triterpenoids
10 mg of the extract was dissolved in 1 ml of chloroform and 1 ml of acetic anhydride was added following the addition of 2 ml of concentrated sulphuric acid. Formation of reddish violet colour indicates the presence of triterpenoids.
Test for Glycosides
A small quantity of the extract was hydrolysed with Hydrochloric acid for few hours on a water bath. To the hydrolysate, 1ml of pyridine was added followed by a few drops of sodium nitroprusside solution and it was made alkaline with sodium hydroxide solution. Appearance of pink to red colour shows the presence of glycosides.
Test for Saponins
1 ml of the extract was diluted with 20 ml of distilled water and it was agitated in a graduated cylinder for 15 minutes. The formation of 1cm layer of foam shows the presence of saponins.
Test for Tannins
5 ml of the extract was added with few drops of 1% lead acetate. Formation of yellow precipitate indicates the presence of tannins.
Test for Phenols
1ml of alcoholic extract was added with 2 ml of distilled water followed by a few drops of 10% aqueous ferric chloride solution. Formation of blue or green colour indicates the presence of phenols.
Test for reducing sugars
1 ml of the extract was diluted with 2 ml of distilled water followed by addition of few drops of Fehling’s solution and the mixtures warmed. Brick red precipitate at the bottom of the test tubes indicates the presence of reducing sugars.
Test for amino acids
1 ml of the extract was treated with few drops of Ninhydrin reagent. Appearance of purple colour shows the presence of amino acids.
Tests for Gums and Mucilages
10 ml of extract was slowly added to 25 ml of absolute alcohol under constant stirring. Precipitation indicates the presence of gums and mucilages.
High performance thin layer chromatography (HPTLC)
A densitometric HPTLC analysis was performed for the development of characteristic finger printing profile. The E.alsinoides L. methanolic extract was dissolved with HPLC grade methanol 100 mg/0.5 ml. The solution was centrifuged at 3000 rpm for 5 min and collected the supernatant liquid, which was used as test solution for HPTLC analysis. 5 µl of Test solutions were loaded as 8mm band length in the 10 x 10 Silica gel 60F254 TLC plate using Hamilton syringe and CAMAG LINOMAT 5 instrument. The samples loaded plate was kept in TLC twin trough developing chamber (after saturated with Solvent vapor) with respective mobile phase (Polyphenolic compound) and the plate was developed in the respective mobile phase [Toluene-Chloroform-Acetone (4 : 2.5 : 3.5)] up to 90 mm. The developed plate was dried by hot air to evaporate solvents from the plate. The plate was kept in Photo-documentation chamber (CAMAG REPROSTAR 3) and captured the images at White light and UV 254 nm. The developed plate was sprayed with respective spray reagent and dried at 100ºC in Hot air oven. The plate was photo-documented in Daylight mode using Photo-documentation chamber. Finally, the plate was fixed in scanner stage and scanning was done at 500 nm. The Peak table, Peak display and Peak densitogram was noted [18].
Results and Discussion
Phytochemical analysis
Table 1: Phytochemical screening of Evolvulus alsinoides L. leaf extract
| Phytochemicals | Inference |
| Flavonoids | +++ |
| Alkaloids | +++ |
| Steroids | + |
| Triterpenoids | ++ |
| Glycosides | ++ |
| Saponins | ++ |
| Tannins | ++ |
| Phenols | +++ |
| Reducing sugars | + |
| Aminoacids | + |
| Gums and Mucilages | + |
+ = present, ++ = moderately present, +++ = Appreciable amount
A variety of herbal extracts contain different phytochemicals with biological activity that can be of valuable therapeutic index. Much of the protective effect has been attributed by phytochemicals. Qualitative analysis of methanolic extract of the leaves of E.alsinoides L. showed the presence of major groups of phytochemical constituents (Table 1). Phytochemical screening revealed the presence of flavonoids, steroids, glycosides, saponnins, phenols, aminoacids (proteins), reducing sugars (carbohydrates), alkaloids, tannins, triterpenoids, gums and mucilages in E.alsinoides L. extract. From the degree of colour change, it was found that the flavonoids, phenols and alkaloids are present in high concentration. The other secondary metabolites like glycosides saponins, tannins, triterpenoids are present in moderate amount and aminoacids, reducing sugars, steroids, gums and mucilages are found to be present in less concentration.
Different phytochemicals have been found to possess a wide range of activities, which may help in protection against chronic diseases. Phenolic compounds exist widely in plants. They have a significant role as defense compounds and are known to be important in the survival of a plant in its environment [19]. The phenolic compounds such as flavonoids, phenolic acids and tannins are considered to be major contributors to the antioxidant capacity of plants. The diverse biological activities may be related to their antioxidant activity [20]. The phenolics constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators [21].
Phytochemicals such as saponins, terpenoids, flavonoids, tannins, steroids and alkaloids have anti-inflammatory effects [22, 23]. Presence of tannins suggests the ability of this plant to play a major role as antihaemorrhagic agent and has been shown to have immense significance as antihypercholesterol, hypotensive and cardiac depressant properties [24]. Glycosides, flavonoids, tannins and alkaloids have antimicrobial [25], hypoglycemic activities [26]. It has been reported that saponins possess hypocholesterolemic and antidiabetic properties [27]. Steroids, saponins and triterpenoids showed the analgesic properties [28, 29] and are responsible for central nervous system activities [30].
HPTLC fingerprinting
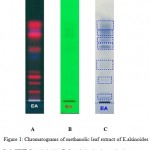 |
Figure 1: Chromatograms of methanolic leaf extract of E.alsinoides L. in HPTLC analysis (A) Before derivatization under day light (B) Under UV 254 nm (C) After derivatization under day light |
 |
Figure 2 : (a) Baseline display (Scanned at 500nm) (b) Peak densitogram display.
|
Table 2: Peak table with Rf values, height and area of polyphenols
| Track | Peak | Rf | Height | Area | Assigned substance |
| EA | 1 | 0.04 | 86.3 | 1080.6 | Polyphenol 1 |
| EA | 2 | 0.21 | 53 | 1139.3 | Polyphenol 2 |
| EA | 3 | 0.33 | 52.7 | 1330.4 | Polyphenol 3 |
| EA | 4 | 0.36 | 50.5 | 2177 | Polyphenol 4 |
| EA | 5 | 0.52 | 239.5 | 8876 | Polyphenol 5 |
| EA | 6 | 0.58 | 193.6 | 6422.4 | Polyphenol 6 |
| EA | 7 | 0.66 | 257.2 | 15317.9 | Polyphenol 7 |
| EA | 8 | 0.70 | 215.9 | 14080.9 | Polyphenol 8 |
HPTLC is a well defined analytical technique based on thin layer chromatography, but with enhancements intended to increase the resolution of the compounds to be separated and to allow quantitative analysis of the compounds. HPTLC method has been found to be rapid, sensitive, precise, and accurate and it has been applied for simultaneous quantitation of phytoconstituents [31]. HPTLC fingerprinting of methanolic leaf extract of E.alsinoides revealed the presence of phenolic compounds. The corresponding HPTLC chromatograms are presented in Figure 1 and 2. The peak height, area and Rf values are shown correspondingly in Table 2. Blue-grey colored zone detected in UV after derivatization in the chromatogram (Figure 1C) confirms the presence of polyphenols. The extracts were run along with the standard polyphenol compounds. HPTLC profile showed the separation of polyphenols each having different retention factor. The leaf extract shows the presence of polyphenols in the chromatograph as well as in UV after derivatization. The Rf value was found to be 0.04, 0.21, 0.33, 0.36, 0.52, 0.58, 0.66, 0.70 of peak 1, 2, 3, 4, 5, 6, 7, 8 respectively. All the peaks were found as polyphenols. The peak height of the respective polyphenols was also given in the Table 2.
The principles of TLC and HPTLC methods are identical, but because of the use of kinetically optimized fine-particle layers in HPTLC, separation is faster and more efficient and the results are more reliable and reproducible. In combination with digital scanning profiling, HPTLC provides accurate, precise Rf values and quantitative analysis of sample constituents by in situ scanning densitometry, as well as a record of the separation in the form of a chromatogram with fractions represented as peaks with defined parameters. The pictorial fluorescence image of HPTLC is more attractive to herbal analysts for constructing a herbal chromatographic fingerprint by means of HPTLC [32]. These images coupled with the scanning profiles provided adequate information and parameters for comprehensive identification, assessment and comparison of major active constituent fingerprints in the samples studied to serve as a basis for their use in medicinal preparations.
Conclusion
The preliminary screening tests may be useful in the detection of the bioactive principles and subsequently may lead to the drug discovery and development. Further, these tests facilitate their quantitative estimation and qualitative separation of pharmacologically active chemical compounds. The phytochemical screening and chromatographic analysis shows that the methanol extracts of Evolvulus alsinoides Linn. leaves are rich in phytochemical constituents and polyphenols. The presence of these phytochemicals is an indicator that the plant can be a potential source of precursors in the development of synthetic drugs. The data generated from these experiments have provided the chemical basis for the wide use of this plant as therapeutic agent for treating various ailments. However, there is a need to further carry out advanced spectroscopic studies in order to elucidate the structure of these compounds and to understand the possible mechanisms involved in the use of this plant in the ethno medical practices.
References
- Dursum, E., Otles, S., and Akcicek, E. “Herbs as food source in Turkey”. Asian Pacific J. Cancer Prev, 5:334-339 (2004).
- Rout, S.P. Choudhary, K.A. Kar, D.M. Das, L., and Jain, A. “Plants in traditional medicinal
- system-future source of new drugs”. Internl. J. Pharmacy & Pharmaceurical Sci. 1 (1):1-23 (2009).
- Chidambara, K., Vanitha, A., Mahadeva, M., Ravishankar, G. “Antioxidant and antimicrobial activity of Cissus quandrangularis L”. J. of medicinal food, 6:2 (2003).
- Duraipandiyan, V., Ayyanar, M., Ignacimuthu, S. “Antimicrobial Activity of Some Ethnomedical Plants Used by Paliyar Tribe from Tamil Nadu, India”. BMC complementary and alternative medicine. 635 (2006).
- Austuin, D.F. “Evolvulus alsinoides (Convolvulaceae): An American herb in the Old World”. J Ethnopharmacol. 22:713 (2008).
- Goyal, P.R. Singh, K.P. “Shankhpuspi (Evolvulus alsinoides Linn.): a medicinal herb”. Int J Mendel. 22:124 (2005).
- Rajaqkaruna, N., Harris, C.S. Towers, G.H.N. “Antimicrobial activity of plants collected from Serpentine outcrops in Sri Lanka”. Pharm Biol. 40:235-244 (2002).
- Ayyanar, M., Ignacimuthu, S. “Traditional knowledge of Kani tribals in Kouthalai of Tirunelveli hills, Tamil Nadu, India”. J Ethnopharmacol.102:246-255 (2005).
- Siripurapu, K.B. Gupta, P., Bhatia, G., Maurya, R., Nath, C., Palit, G. “Adaptogenic and anti-amnesic properties of Evolvulus alsinoides in rodents”. Pharmacol Biochem Behav. 81:424-443 (2005).
- Tharan, N.T. Vadivu, R., Palanisamy, M., Justin, V. “Antibacterial Activity of Evolvulus alsinoides”. Indian Drugs. 40:585-586 (2003).
- Purohit, M.G. Shanthaveerappa, B.K. Badami, S., Swamy, H.K.S. Shrishailappa, B. “Antiulcer and anticatatonic activity of alcoholic extract of Evolvulus alsinoides (Convolvulaceae)”. Ind J Pharma Sci. 58:110-112 (1996).
- Auddy, B. “Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases”. J Ethnopharmacol. 84:131-138 (2003).
- Ganju, L., Karan, D., Chanda, S., Srivastava, K.K. Sawhney, R.C. Selvamurthy, W. “Immunomodulatory effects of agents of plant origin”. Biomed-Pharmacother. 57:296-300 (2003).
- Dash, D.K. Yeligar, V.C. Nayak, S.S. Ghosh, T., Rajalingam, D., Sengupta, P., Maiti, B.C. and Maity T.K. “Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R.Br. on paracetamol induced hepatotoxicity in rats”. Trop J Pharm Res. 6(3):755-765 (2007).
- Ratnasooriya, W., Hewageegana, H.G.S.P. Jayakody, J.R.A.C. Ariyawansa, H.A.S. Kulatunga, R.D.H. “Gastroprotective activity of Evolvulus alsinoides L. powder”. Aust J Med Herbalism. 17: 55-60 (2005).
- Harborne, J.B. “Phytochemical methods”. A guide to modern techniques of plant analysis. 3rd edn. Chapman and Hall Int. Ed., New York, (1998).
- Kokate, C.K. Pharmacognosy. 16th edn., Nirali Prakashan, Mumbai, India, (2001).
- Shah, C.R. Suhagia, B.N. Shah, N.J. Patel D.R. and Patel, N.M. “Stability-indicating simultaneous HPTLC method for olanzapine and fluoxetine in combined tablet Dosage form”. Indian J. Pharmaceutical Sci., 70(2): 251-255 (2008).
- Puupponen-Pimia, R., Nohynek, L., Schmidlin, S., Kahkonen, M., Heinonen, M., Maatta- Riihinen, K., and Oksman-Caldentey, K.M. “Berry phenolics selectively inhibit the growth of intestinal pathogens”. J. Appl. Microbiol. 98, 991 (2005).
- Chung, K. T. Wong, T. Y. Huang, Y. W. Lin, Y. “Tannins and human health: a review”. Crit Rev food sci nutr. 38, 421- 464 (1998).
- Cao, G., Sofic, E., Prior, R.L. “Antioxidant and prooxidant behavior of flavonoids: Structureactivity relationships”. Free Radic. Biol. Med. 22, 749-760 (1997).
- Akindele, A.J. and Adeyemi, O.O. “Antiinflammatory activity of the aqueous leaf extract of Byrsocarpus coccineus”. Fitoterapia, 78: 25-28 (2007).
- Ilkay Orhan, Esra Kupeli, Bilge Sener, and Erdem Yesilada. “Appraisal of anti – inflammatory potential of the clubmoss, Lycopodium clavatum L”. J. Ethnopharmacol., 109: 146-150 (2007).
- Price K.R. Johnson, T.I. Fenwick, G.R. “The Chemistry and Biological Significance of Saponins in Food and feeding stuffs”. Crit Rev Food Sci Nutr., 26:22-48 (1987).
- Abo, K.A. Ogunleye, V.O. Ashidi, J.S. “Antimicrobial Potential of Spondias mombin, Croton zambesicus and Zygotritoma crocea”. Phytothe. Res.13: 494-497 (1999).
- Cherian, S., and Augusti, K.T. “Insulin sparing action of leucopelargonidin derivative isolated from Ficus bengalesis Linn”. Indian J. Exp. Biol., 33: 608-611 (1995).
- Rupasinghe, H.P. Jackson, C.J. Poysa, V., Di Berado, C., Bewley J.D. and Jenkinson, J. “Soyasapogenol A and B distribution in Soybean (Glycine Max L.Merr) in relation to seed physiology, genetic variability and growing location”. J. Agric. Food Chem., 51: 5888-5894 (2003).
- Sayyah, M., Hadidi N., and Kamalinejad, M. “Analgesic and anti-inflammatory activity of Lactuca sativa seed extract in rats”. J. Ethnopharmacol., 92:325-329 (2004).
- Malairajan, P., Geetha Gopalakrishnan, S., Narasimhan and Jessi Kala Veni, K. “Analgesic activity of some Indian medicinal plants”. J. Ethnopharmacol., 19: 425-428 (2006).
- Argal, A., and Pathak, A.K. “CNS activity of Calotropis gigantea roots”. J. Ethnopharmacoogyl., 106: 142-145 (2006).
- Sutar, A. C. Sohoni, D. P. Banavaliker, M.M. Blyani, M. K. “HPTLC methods for quantitative estimation of genistein and diadzein with its Glycosides in Glycine max”. Indian drugs. 39, 434 (2002).
- Di, X., Chan, K.K. Leung, H.W. Huie, C.W. “Fingerprint profiling of acid hydrolyzates of polysaccharides extracted from the fruiting bodies and spors of Lingzhi by high-performance thin layer chromatography”. J Chromatogr A., 1018: 85 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.





