Manuscript accepted on : April 16, 2010
Published online on: 28-12-2010
Large Scale Production of Oxytetracyclin Using Streptomyces Varsovances
Abhishek Mule*, R.C. Patil1*, Ujwala Jadhav2, V.I. Katchi3, Asha J. Rao3 and S.K. Gaikwad4
1Department of Microbiology, Bhavan’s College, Andheri (W), Mumbai - 400 058 (India). 2Department of Life Science, University of Mumbai, Santa Cruz (E), Mumbai - 400 098 (India). 3Department of Zoology, Bhavan’s College, Andheri (W), Mumbai - 400 058 (India). 4Department Of Cell and Molecular Biology, Rajiv Gandhi Institute of IT and Biotechnology Bharati Vidyapeeth University (India).
ABSTRACT: Oxytetracyclin production by Streptomyces varsovances was done using sweet potato mash medium. Important process parameters like optimum pH ,temperature, tip speed and Kla value for the antibiotic production was determined. Extraction of oxytetracyclin was carried out using boron tetrafluoride, extracted antibiotic was estimated using bioassay and HPLC. New production method was effective and gave 25% more yield as compared to traditional methods, extraction method is promising for large scale production.
KEYWORDS: Oxytetracyclin; pH; temperature; tip speed; boron tetrafluoride
Download this article as:| Copy the following to cite this article: Mule A, Patil R. C, Jadhav U, Katchi V. I, Rao A. J, Gaikwad S. K, Mammen A. Large Scale Production of Oxytetracyclin Using Streptomyces Varsovances. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Mule A, Patil R. C, Jadhav U, Katchi V. I, Rao A. J, Gaikwad S. K, Mammen A. Large Scale Production of Oxytetracyclin Using Streptomyces Varsovances. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9294 |
Introduction
It has been well established that microorganisms are a virtually unlimited source of natural products, many of which have potential therapeutic applications. Filamentous soil bacteria of the genus Streptomyces are remarkable, and merit special consideration with regard to the morphological and metabolic differentiation phenomena they manifest during later stages of development. Streptomyces species generally synthesize a sizeable number of diverse natural secondary metabolites, the best known of which are antibiotics currently used worldwide as pharmaceutical and agrochemical products (El-Naggaret al., 2003; Pamboukian and Facciotti, 2004; Ben-Fguiraet al., 2005). Two-thirds of commercially available antibiotics (Miyadoh, 1993) and approximately 60% of those used for agricultural purposes were isolated originally from Streptomyces species (Tanaka and Mura, 1993).
In this paper oxytetracyclion production was studied in Streptomyces varsovances culture, heat shocked culture was further used for large scale production of oxytetracyclin, different parameter in 4 liter scale up process were determined in antibiotic production, using sweet potato mash medium, optimum pH, tip speed was determined in antibiotic production. Additionally, the medium was extracted by novel method using ionic liquid.
Tetracyclines are broad-spectrum antibiotics, and the hydrocarbon derivatives of octahydronaphthacene with four annelated 6-membered rings. Submerged culture was usually used for tetracycline production, resulting in much energy input and waste water production.324I n addition, culture media and culture conditions would affect the kind and the quantity of antibiotic production.”’ Sweet potatoes and its residue are abundantly available. Residue of starchy materials has a high productivity per hectare and an excellent rate of fermentation by a great number of fast-growing microorganisms. With the goal of being economically competitive, sweet potato residue, a starchy agricultural waste, was used in this article to produce oxy-tetracyclines with Streptomyces viridifuciens by submerge fermentation.
Material and Methods
Sweet Potato Residue
Sweet potato residue was purchased from the local market in Taiwan, screened with 4-16 mesh to remove the dust and large aggregates. It contains 14.0-16.1% moisture, 2.32% crude protein, 3.6% ash, 18.1% crude fiber, and 65.4% carbohydrate.
Estimation of starch
Estimation of starch was carried out by the method of McCready et al. (1950). The residual mass obtained during fermenatation was suspended in 5.0 ml of water and subsequently 6.5 ml of 52% perchloric acid was added to the residue after stirring of the mixture, the contents were centrifuged for 20 minutes at 2000 rpm. The supernatant was decanted and collected and the whole procedure was repeated thrice. Supernatant of each step were then poured and the total volume was made up to 100 ml with distilled water. The mixture was then filtered through whatmanfilterpaper (No.42) 1.0 ml of aliquot of this filtrate was analyzed for starch content following the same procedure as that of total soluble sugar. Quantity of starch was calculated in terms of glucose equivalent and factor 0.9 was used to convert the values of glucose to starch. The quantity of starch was expressed in terms of mg/g fresh wt. of tissue.
Tested Organisms
Streptomyces varsovancessATCC 11989 was used for tetracycline and chlortetracycline production, and Bacillus subtilisATCC 6633 was used for bioassay of antimicrobial activity.
Instruments
All operations were performed on Sartorius Stadium BiostatA Plus fed batch semi-continuous reactor.
Culture Media and Culture Conditions
The tested organism was inoculated in inoculum flask (of the culture 1.2 X 105 cells / ml) containing, the medium constituting soluble sweet potato starch 10 g, (NH)4SO4, 2.0 g, CaCO3, 2.0 g, NaCl 1.0 g, K2HP04, 1.0 g, MgS04-7H20 1.0 g, trace-element solution 1 mL (contained FeS04.7H,0 1.0 g, CuSO4,. 5H20 0.5 g, ZnS04.7H20 1.0 g and MnS04.2H20 0.1 g/L) in the hot water bath maintained at desired temperature.
Extraction of Antibiotics
Ionic liquid BF4 of 1.5 mL, NaH2PO4 (37%) of 1.5 mL and hydrochloric acid tetracycline of 1.8 mL were added in a 10 mL centrifuge tube. Na2HPO4-H3PO4 was used to adjust the pH value of the above solution. After super-sonic oscillation for 3 min, the mixture was settled for a moment. Low speed centrifuge was used to separate the mixture. After separation, the ionic liquid was taken out and diluted with water to 5 mL, and the absorption spectrum was determined.
Qualitative and Quantitative Determination of Antibiotics Bioassay
Antimicrobial activity of culture extracts was measured by the sterile paper disc method (diameter 8 mm, Hi Media laboratory Limited.) with B. subtilisATCC 6633 as the tested organism in antibiotic medium I (Difco Laboratory, USA) at 30°C. Total tetracycline equivalent potency was calculated from the clear zone of a standard curve of tetracycline (Sigma Co., USA) in the range of 1 pg/mL to 1 X 105pg/mL
High Performance Liquid Chromatography Method
Culture extract was filtered with 0.45 pm Millipore filter (Millipore Ltd. India), and antimicrobial activity was determined by Shimadzu LC-5A Liquid Chromatograph. The operation conditions were with column ODS 120 T and mixture of methanol: acetonitrile: water: pH 3.5 0.2M phosphate buffer at ratios of 10:20:60: 10 as the mobile phase. The flow rate was 1 mL/min, and the antibiotic was detected with UV detector at wavelength 254 nm or 355 nm. Authentic oxytetracycline was used as the standard for qualitative and quantitative determination in the range of 1 pg/mL to 120 pg/mL.
Results and Discussion
The tetracycline fermentation process is characterized by the complex dynamics and highly interlinked sub-processes of the biological transformations. Reliable monitoring and control of the industrial production of the tetracycline requires quality information on the process in real time, which can significant upgrade the production capability. There are many measurement techniques, which enable gathering the directly or indirectly measured physical, chemical and biological parameters of the process. However, due to limiting factors, such as reliability, risk of medium contamination, difficulties in transferring the results from pilot plants to industrial fermentors and complexity of the process, the real-time optimization of the industrial fermentation process is not an easy task. On-line measurements of oxygen and carbon dioxide concentrations in the fermentor’s exhaust gas and reaction heat flow were conducted during tetracycline fermentation in an industrial 80 m3 batch reactor. The concentration of oxygen in the fermentor’s exhaust gas was measured using an IJS MK 100 gauge operating on the principle of oxygen diffusion between actual and reference states. In order to determine the reaction heat flow the following process parameters were measured on-line: the inlet and the outlet temperature of the cooling water; the inlet and the outlet air temperature; the temperature of the broth in the fermentor using sterilisable Pt-100 probes; the flow of cooling water through the calibrated valve; the flow of air using a HeinrichsMesstechnik TSK-200 meter; the humidity of the inlet and the outlet air using a Vaisala HMP 234 meter; and the power of the mixer using the electric power consumed by the driving electro-motor and the known efficiency of the transmission and the rotor. The biomass concentration in the broth cX was measured off-line with the ‘spread plate count’ method7 in three parallel runs.
Oxygen in exhaust gas was measured using formula
Where is the oxygen mass ratio with regard to the liquid volume in the
fermentor
The oxygen uptake rate (OUR) can be defined as
Fermentor was maintained at 250 C, dissolved oxygen concentration was determined by using dynamic method of gassing out technique, as per Taguchi and Humphrey results of which has been showed in Fig. 1.1
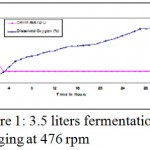 |
Figure 1: 3.5 liters fermentation air sparging at 476 rpm.
|
Fig. 1.1 A shows, maximum saturation of oxygen (100%), at o hours, however it decreases steadily over a period of time at the end of five hour it became 20%, which was further maintained by starting sparging and aeration at 476 rpm at 4th our of fermentation.
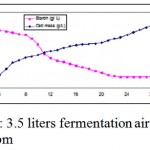 |
Figure 2: 3.5 liters fermentation air sparging at 476 rpm.
|
Estimation of starch was carried out by the method of McCreadyet al. (1950). Initial starch concentration was found to be 22 g/L, which decreased steadily over a period of time, at the end of 26 hours fermentation the starch concentration was 2.5 g . This was found to be comparable with Saleem et al 2009 , in which Streptomyces species gave better yield using Starch- Casein medium, additionally its was also found comparable with Kanda et al 2010, in which echinocandin type antibiotic FR901379 was produced using starchy substrates, this is also in accordance with Yang and Ling 1988, in which Sweet potato was used as a substrate for tetracycline production. Asagbra et al 2005 also proposed antibiotic production by Streptomyces sp. OXC1 using starchy agricultural waste.
Extraction of Antibiotic
Antibiotic extraction was done using method of Chun-hong et al 2009, in which ionic liquid like was used , ionic liquid are non-flammable, highly conductive, stable reagents for many organic and inorganic analyses have a good solubility. The application of ionic liquids in separation and chemical reactions shows good prospects. Ionic liquid is an ideal substitute for the traditional volatile solvents and an environmentally friendly liquid. It is a new generation of “green” and “design” solvent in chemical researches and its chemical production has received more and more attention.
Monitoring tetracycline production
Time course of tetracycline potency has been monitored and plotted in Fig. 1.3, No tetracycline was produced till end of ten hours; product formation was produced after ten hours, which gradually increase as time elapsed maximum production of tetracycline was reported at the end of 29 hours. This results were found in comparison with Anisha and P. Prema 2008, in which immobilized Streptomyces griseoloalbus yielded 123 mcg/ml of the tetracycline.
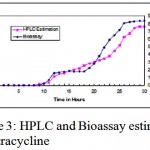 |
Figure 3: HPLC and Bioassay estimation of Tetracycline.
|
Parameter studies
pH of the medium
Optimum pH for the production of tetracycline was determined medium pH was varied between 4 to 12 pH 6 was found to be optimum for antibiotic production. (Fig. 1.4).this was in the agreement of Zaria 1993, who reported antibiotic production at pH 6 using Coagulase-negative Staphylococci, additionally this is in accordance with findings of Paul and Banerjee 1983, in antibiotic production from Streptomyces galbus.
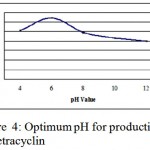 |
Figure 4: Optimum pH for production of oxytetracyclin.
|
Temperature of the medium
Optimum temperature for the production of tetracycline was determined medium temperature was varied between 25oC to 45 oC, 30 oC was found to be optimum for antibiotic production. (Fig. 1.5).this was found to be in accordance with Atta et al., 2009, who studied taxonomy, fermentation, purification and biological activities of sparsomycin antibiotic production by Streptomyces sp. AZ-NIOFD1.
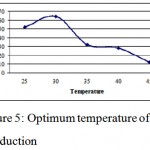 |
Figure 5: Optimum temperature of production
|
Tip speed of the impeller
Effect of impeller tip speed was determined on the production of oxyteracyclin increase in tip speed from 5000 rpm to 10000 rpm showed a general trend to increase in o oxyteracyclin production however increasing speed to 12000 rpm did not give appreciable increase in antibiotic yield. (Fig. 1.6)
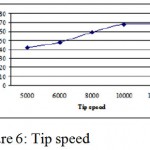 |
Figure 6: Tip speed
|
Oxytetracyclin production was carried out in non conventional medium; effect of different parameter was studied. The medium and extraction procedure was promising and can be exploited for commercial scale production.
References
- El-Naggar, M.Y., Hassan M.A., Said W.Y., and. El-Aassar S.A., J. Gen. Appl. Microbiol. 49: 235-243 (2003).
- Pamboukian, C.R.D. and. Facciotti. M.C.R Process Biochem.39: 2249-2255 (2004).
- Ben-Fguira, L.F., Fosto S., Mehdi R.B., Mellouli L., and LaatschH..Microbiol. Res. 156: 341-347 (2005).
- Miyadoh, S. Actinomycetologica. 9: 100-106 (1993)
- Tanaka, Y. T. and. Mura S.O. Ann. Rev. Microbiol. 47: 57-87 (1993)
- Mccready, R. M., Guggolz, J., Silviera, V., and Owens, H. S..Anal. Chem. 22: 1156 (1950)
- Saleem TSM, Ravi V, Gauthaman K, Saisivam S., J. Pharm. Sci. & Res. 1(2): 57-60 (2009)
- Kanda1 M, Yamamoto E, Hayashi A, Yabutani1 T, Yamashita M and Honda H. J of Bio. andBioeng. 109(2): 138-144 (2010)
- Shang-Shyng Yang and Meei-Yueh Ling Biotech.andBioeng.,33: 1021-1028 (1989).
- Agnes E. Asagbra, Abiodun I. Sanni and Olusola B. Oyewole, Solid-state fermentation production of tetracycline by Streptomyces strains using some agricultural wastes as substrate World Journal of Microbiology and Biotechnology, 21(2): (2005).
- Chun-hong MA, Liang W, Yong-sheng1, Guang-bo, YIN Yan-su WANG Ren-zhang3 and LI Dong- CHEM. RES. CHINESE UNIVERSITIES, 25(6): 832-835 (2009).
- G.S. Anisha and P. PremaBiores.Technol.99(9) (2008).
- Zaria L T., Antibiotic Production by Coagulase-negative Staphylococci Isolated from the Skin of Pigs Microbial Ecology in Health and Disease, 6(3): 123-127 (1993).
- Paul K., and Banerjee A. K., Folia Microbiologica28-5 (1983).
- Atta H.M., Dabour S.M. and Desoukey S.G., Sparsomycin Antibiotic Production by Streptomyces Sp. AZ-NIOFD1: taxonomy, fermentation, purification and biological activities American-Eurasian J. Agric. & Environ. Sci., 5(3): 368-377 (2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.





