Manuscript accepted on : December 13, 2010
Published online on: 28-12-2010
Adil A. Wani*, M. Sikdar-Bar, H. A. Khan and P. A. Pervaiz
Department of Zoology, Dr. H.S. Gour University, Sagar - 470 003 India.
Corresponding Author E-mail:waniadil@rocketmail.com.
ABSTRACT: The metals are discharged into aquatic bodies from many sources, often at sublethal concentrations. The hazardous effect of sublethal concentrations (2 and 5 mgl-1) of copper sulphate on liver histology of African catfish, Clarias gariepinus was analyzed histologically after 30 and 60 days of exposure. The control group was also maintained simultaneously. The liver of control group showed normal histo-archetechture, while as copper sulphate exposed fish showed cytoplasmic vacuolation, nuclear degeneration, focal areas of necrosis, hypertrophy of hepatocytes, pycnotic nuclei haemorrhage and haemolysis due to rupture of blood vessels. This research reveals that copper sulphate has a deleterious impact on liver histopathology of Clarias gariepinus, which could be a suitable biomarker for environmental contaminations.
KEYWORDS: Copper sulphate; Clarias gariepinus; histopathology; liver
Download this article as:| Copy the following to cite this article: Wani A. A, Sikdar-Bar M, Khan H. A, Pervaiz P. A. Histopathological Changes Induced In Liver by Long-Term Exposure to Sublethal Concentrations of Copper Sulphate in the African Catfish, Clarias Gariepinus (Burchell, 1822). Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Wani A. A, Sikdar-Bar M, Khan H. A, Pervaiz P. A. Histopathological Changes Induced In Liver by Long-Term Exposure to Sublethal Concentrations of Copper Sulphate in the African Catfish, Clarias Gariepinus (Burchell, 1822). Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9793 |
Introduction
The aquatic environment is crucial for the continued survival of life on earth. However, an intense activity in industrialization and agricultural sectors has increased the levels of heavy metals in aquatic environment1. Mining of metal ores and coal are main contributors to heavy metal pollution by discharging contaminated drainage into water bodies2.
Copper is an essential micronutrient as it is the essential component of numerous enzymatic systems and liver proteins homocuprien and heptacuprien3. Copper sulphate is used as an agrochemical and in aquaculture for treatment of bacterial and fungal diseases of fish. In spite of its essential functions it can cause adverse toxicological effects on fish if the dose increased to be more than the permissible concentration limit. The cupric copper (Cu+2) is most toxic state and is most common than other oxidation states of copper in aquatic bodies.
The liver plays not only important function in the metabolism and excretion, but is a major site for detoxification of xenobiotics chemicals and heavy metals. Thus, liver is the primary target of toxic substances which lead to histopathological changes and malfunction of enzymes. Histopathological assays provide a valuable screening method for evaluation of fish health exposed to contaminates4, both in laboratory 5 and field studies6, 7.
The present study aimed to evaluate the histopathological changes induced by long-term sublethal exposure of copper sulphate in the liver of African catfish (Clariasgariepinus) under experimental conditions.
Materials and Methods
Fish and experimental system
Healthy adult specimensofClariasgariepinus (110-110g body weight, 18-20cm in length)were collected from a single population from local fish market at Bhopal and were brought to the laboratory. They were kept in transparent glass aquariums to observe any visible pathological symptoms. Before introducing in the aquarium fishes were treated with 0.1% KMnO4 solution to obviate any dermal infection. Fishes were acclimatized to laboratory conditions for a period of 15 days. Coppersulphate (Ranbaxy, India) was used for the preparation of concentrations (stock solution) by adopting the dilution techniques. The fishes were divided into three groups kept in three glass aquariums (200l), group A and B were treated with coppersulphate concentrations of 2 and 5 mg/l,respectively. Group C served as the control and all the three groups were fed with chopped meat once daily throughout the experimental period.
Histological procedure
On the 30 and 60 days of the exposure of two different sub-lethal concentrations of coppersulphate, one fish form each exposed group and control group were sacrificed by giving a sharp blow on head and dissected out. Liver was removed and washed in saline water to remove blood and fixed in aqueous Bouin’s fixative for 24 hrs. They were then dehydrated through graded series of ethanol and embedded in paraffin wax(M.P. 58-680C). Blocks were prepared and sectioned at a thickness of 6-7 microns. The sections were deparaffinized in xylene and stained with Ehrlich’s haematoxylin-eosin (HE). Histopathological changes induced by CuSO4 exposure in the liver were analyzed and photographed under photomicroscope (Olympus) along with control group.
Results
Liver
Control group
The liver after 30 and 60 days exhibited normal histology with polyhedral hepatocytes containing centrally located spherical nucleus with homogeneous deeply stained cytoplasm. In between hepatic cells, bile passage can be observed (Fig. 1)
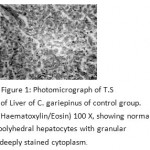 |
Figure 1: Photomicrograph of T.S of Liver of C. gariepinus of control group. (Haematoxylin/Eosin) 100 X, showing normal polyhedral hepatocytes with granular deeply stained cytoplasm. |
Treated groups
2 mg of CuSO4/l after 30 and 60 days
After 30 days degeneration of hepatic cells and blood congestion in vessels were observed.Atrophy of hepatocyteswas reported (Fig. 2). After 60 days of exposure cytoplasm appeared reticulated and was mostly occupied by large vacuoles.Hypertrophy of hepatic cells was clearly seen. Haemorrhage and haemolysis was observed due to rupture of blood vessels. Hepatocytes were damaged around the blood vessels (Fig. 3)
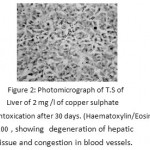 |
Figure 2: Photomicrograph of T.S of Liver of 2 mg /l of copper sulphate intoxication after 30 days. (Haematoxylin/Eosin) 100 , showing degeneration of hepatic tissue and congestion in blood vessels. |
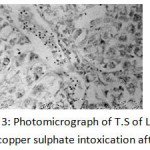 |
Figure 3: Photomicrograph of T.S of Liver of 2 mg /l of copper sulphate intoxication after 60 days. (Haematoxylin/Eosin) 100X, showing reticulated cytoplasm of hepatocytes with vacuoles, hypertrophy of hepatocytes, Haemorrhage and haemolysis of blood vessels. |
5 mg of CuSO4/l after 30 and 60 days
After 30 days hepatocytic nuclei were irregular in shape and become pycnotic.Shifting of the nuclei towards the periphery of the hepatocytes was seen (eccentric nuclei). Nuclear degeneration was also noticed in few hepatocytes. Necrosis of hepatic tissue andenlargement of bile passages were prominent.Cell membrane of the hepatocytes was reported to be ruptured and showing synctial appearance(Fig. 4). Extensive cytoplasmic vacuolation and focal necrotic areas were observed after 60 days. (Fig. 5)
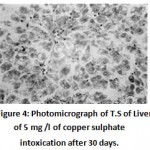 |
Figure 4: Photomicrograph of T.S of Liver of 5 mg /l of copper sulphate intoxication after 30 days. (Haematoxylin/Eosin) 100X, showing nuclear generation, pycnotic, eccentric nuclei with irregular shape and necrosis of hepatic tissue. |
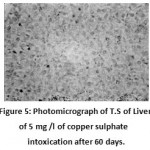 |
Figure 5: Photomicrograph of T.S of Liver of 5 mg /l of copper sulphate intoxication after 60 days. (Haematoxylin/Eosin) 100X, showing cytoplasmic vacuolation and focal necrotic area. |
Discussion
Histopathological studies are considered as direct evidence referring to any adverse effect on fish. Generally, the liver is considered as the principle organ of biotransformation, accumulation, excretion of toxic chemicals. Hepatocytes may thus be expected to be the primary target of toxic substances, providing an excellent biomarker of aquatic pollution8, 9, 10, 11.
In the present study Clarias gariepinus fishfrom control groupshowednormal histo-archetechture with polyhedral hepatocytes having homogeneous deeply stained cytoplasm. Our observations on control fish correspond with the reports of researchers12, 13,14.
The major histopathological abnormalities identified within the liver in this study due to copper sulphate exposure were nuclear degeneration, cytoplasmic vacuolation and hypertrophy of hepatocytes, haemolysis and rupture of blood vessels, necrosis of hepatic tissue, which showed a progressive histological distortion at varied CuSO4 concentrations and period of exposures which is in agreement submission of15. The alterations in size and shape of the hepatocyte nucleus can be regarded as signs of increased metabolic activity due to exposure ofCuSO416.
The rupture of blood vessels and intravascular haemolysis observed may be responsible for the cellular degradation and necrosis in the liver17. Exposure of Oncorynchusmykiss to copper sulphate was found to induce vacuolation of hepatocytes, sinusoidal dilation and congestion in the blood vessels of the liver18, 19, 20. Chronic copper accumulation in the liver of fish causes hepatocytic lysis, cirrhosis and ultimately death21. The hypertrophy is an adaptive alteration of the hepatocytes in response to exposure of toxins22.
The toxic effect of copper is related to its capacity for catalyzing oxidative reactions, leading to the generation of reactive oxygen species23. These highly reactive compounds may also induce tissue alterations and physiological derangement in fish24.
From our results, it can be concluded that the liver histopathological changes in African catfish (Clarias gariepinus) have been related to copper sulphate concentrations and duration of its exposure which can serve as biomarkers of environmental pollution.
Acknowledgements
Authors are thankful to Prof. Mrs. Mangla Bhide, HOD of Zoology, Dr. H.S. Gour University, and Dr. Mrs. M.Sikdar-Bar, Sr. Lecturer,Department of Zoology, Dr. H.S. Gour University, Sagar (M.P) for their valuable guidance for carrying out this work.
References
- Jordao C.P., and Preira M.G., Environ. Monit. Assess. 79, 75-100 (2002).
- Lloyd R., Pollution and freshwater fish, Blackwell scientific Pub. Ltd., London (1992).
- Seymore, M.Sc. Thesis, Rand Afrikaans University, South Africa (1994).
- Hinton D.E and Lauren D.J., American Fish. Society Symposium, 8, 51-66 (1990).
- Jiraungkoorskul W., Sahaphong S. and Kangwaranrangsan N., Environmental Toxicol.,22(1), 92-100 (2007).
- Schwaiger J., Wanke R., Adam S., Pawert M., Honnen W. and Triebskorn R., Aqua. Ecosyst., Stress and Recov.,6, 75-86 (1997).
- Teh S.J., Adams S.M. and Hinton, D.E., Aquat.Toxicol.,37, 51-70 (1997).
- Maurad M., Wahby O., ActaIchthyologica Et Piscatoria. 29(2), 73-79 (1999).
- Dethloff G.M. and Bailey H.C., Toxicol. Chem. 17, 1807-1814(1998).
- Camargo M.M and Martinez C.B., Neotrop. Ichthyol.,5(3),327-336 (2007).
- Atif M. El-Naggar, Soaad A. Mahmoud and Safaa I. T., World J. Fish and Marine Sci., 1(2): 105-114. (2009).
- Hampton, J.A., Lantz R.C., Hinton D.E., J. Anat., 185(1), 58-73 (1989).
- Rocha E., Monteiro R.A. and Pereira C.A., Anat. Rec., 247(3), 317-328 (1997).
- Ongundiran M.A., Fawole, O.O., Adewoye S.O., and Ayandiran T.A., Agric. Biol. J. N. Am., 1(3), 330-342 (2010).
- Figueiredo A., Ferreira J.V., Garcia S., Monnteiro S.M., Carrola J., Matos P., and Fontainhas A., Vet. Bras.,27(3), 103 –109 (2007).
- Paris-Palacios S., Biagianti-Risbourg S. and Vernet G., Aquatic Toxicology. 50, 109-124 (2000).
- Mohamed F.A., World J. Fish and Marine Sci., 1(1), 29-39 (2009).
- Salim S. and Khan S.M., J. Fish., 30, 96-98 (1994).
- Atamanalp M., Turgay S., Fatime G. and Ahmet T., J. of Fish. Aqut. Sci., 3(5), 291-297 (2008).
- Vinodhini R. and Narayanan M., J. Environ. Res., 3(1), 95-100 (2009).
- PourahmadandO’BrienP. J., Toxicol. 143(3), 263 -73,(2000).
- Hinton D.E and Lauren D.J., McCarthy J.F. and Shugart L.R. Lewis Publishers, 17-52 (1990).
- Lopes P.A., Pinheiro T, Santos M.C., Mathias M., Collares-Pereira M.J., Viegas-Crespo A.M., Sci. Total Environ. 280, 153-163 (2001).
- Varanka Z., Rojik I., Varanka I., Nemcsok J. and Abraham M., Comp. Biochem. Physiol., 128, 467-478 (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.





